Efavirenz, emtricitabine and tenofovir disoproxil fumarate: Difference between revisions
No edit summary |
No edit summary |
||
| Line 314: | Line 314: | ||
Emtricitabine is a white to off-white crystalline powder with a solubility of approximately 112 mg/mL in water at 25 °C. | Emtricitabine is a white to off-white crystalline powder with a solubility of approximately 112 mg/mL in water at 25 °C. | ||
Tenofovir Disoproxil Fumarate: Tenofovir DF is a fumaric acid salt of the bis-isopropoxycarbonyloxymethyl ester derivative of tenofovir. The chemical name of tenofovir disoproxil fumarate is 9-[(R)-2 | Tenofovir Disoproxil Fumarate: Tenofovir DF is a fumaric acid salt of the bis-isopropoxycarbonyloxymethyl ester derivative of tenofovir. The chemical name of tenofovir disoproxil fumarate is 9-[(R)-2([bis([(isopropoxycarbonyl)oxy]- methoxy)phosphinyl]methoxy)propyl]adenine fumarate (1:1). It has a molecular formula of C19H30N5O10P • C4H4O4 and a molecular weight of 635.52. It has the following structural formula: | ||
[[File:Tenofovir disoproxil fumarate Structure.png|800px|thumbnail|left|This image is provided by the National Library of Medicine.]] | [[File:Tenofovir disoproxil fumarate Structure.png|800px|thumbnail|left|This image is provided by the National Library of Medicine.]] | ||
Revision as of 15:44, 8 August 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT EXACERBATION OF HEPATITIS B
See full prescribing information for complete Boxed Warning.
LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT EXACERBATION OF HEPATITIS B: Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including tenofovir disoproxil fumarate, a component of efavirenz, emtricitabine, and tenofovir disoproxil fumarate, in combination with other antiretrovirals.
Efavirenz, emtricitabine, and tenofovir disoproxil fumarate is not approved for the treatment of chronic hepatitis B virus (HBV) infection and the safety and efficacy of efavirenz, emtricitabine, and tenofovir disoproxil fumarate have not been established in patients coinfected with HBV and HIV-1. Severe acute exacerbations of hepatitis B have been reported in patients who have discontinued emcitrabine or tenofovir disoproxil fumarate, which are components of efavirenz, emtricitabine, and tenofovir disoproxil fumarate. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who are coinfected with HIV-1 and HBV and discontinue efavirenz, emtricitabine, and tenofovir disoproxil fumarate. If appropriate, initiation of anti-hepatitis B therapy may be warranted.
|
Overview
Efavirenz, emtricitabine and tenofovir disoproxil fumarate is a human immunodeficiency virus nucleoside analog reverse transcriptase inhibitor, human immunodeficiency virus 1 non-nucleoside analog reverse transcriptase inhibitor that is FDA approved for the treatment of HIV-1 infection. There is a Black Box Warning for this drug as shown here. Common adverse reactions include rash, diarrhea, nausea, decreased bone mineral density, dizziness, headache, anxiety, depression, sleep disorder, increased creatine kinase level, sinusitis, upper respiratory tract infection, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Adults and pediatric patients 12 years of age and older with body weight at least 40 kg (at least 88 lbs): The dose of efavirenz, emtricitabine and tenofovir disoproxil fumarate is one tablet once daily taken orally on an empty stomach. Dosing at bedtime may improve the tolerability of nervous system symptoms.
- Renal Impairment: Because efavirenz, emtricitabine and tenofovir disoproxil fumarate is a fixed-dose combination, it should not be prescribed for patients requiring dosage adjustment such as those with moderate or severe renal impairment (estimated creatinine clearance below 50 mL/min).
- Rifampin Coadministration: When efavirenz, emtricitabine and tenofovir disoproxil fumarate is administered with rifampin to patients weighing 50 kg or more, an additional 200 mg/day of efavirenz is recommended
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Efavirenz, emtricitabine and tenofovir disoproxil fumarate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Efavirenz, emtricitabine and tenofovir disoproxil fumarate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Efavirenz, emtricitabine and tenofovir disoproxil fumarate FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Efavirenz, emtricitabine and tenofovir disoproxil fumarate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Efavirenz, emtricitabine and tenofovir disoproxil fumarate in pediatric patients.
Contraindications
Hypersensitivity
ATRIPLA is contraindicated in patients with previously demonstrated clinically significant hypersensitivity (e.g., Stevens-Johnson syndrome, erythema multiforme, or toxic skin eruptions) to efavirenz, a component of ATRIPLA.
Contraindicated Drugs
For some drugs, competition for CYP3A by efavirenz could result in inhibition of their metabolism and create the potential for serious and/or life-threatening adverse reactions (e.g., cardiac arrhythmias, prolonged sedation, or respiratory depression). Drugs that are contraindicated with ATRIPLA are listed in Table 1.
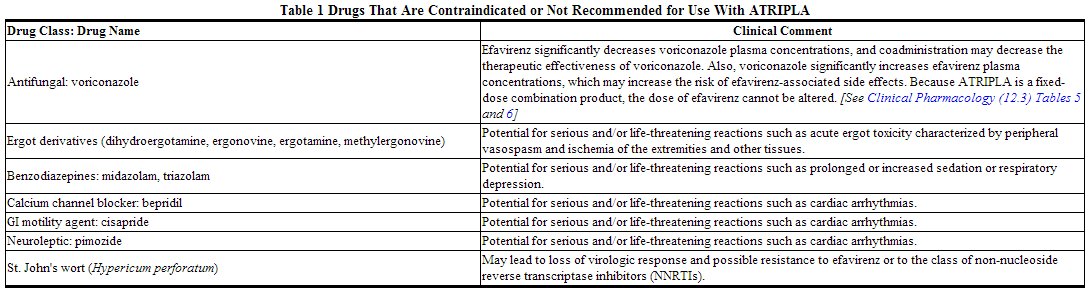
Warnings
|
WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT EXACERBATION OF HEPATITIS B
See full prescribing information for complete Boxed Warning.
LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT EXACERBATION OF HEPATITIS B: Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including tenofovir disoproxil fumarate, a component of efavirenz, emtricitabine, and tenofovir disoproxil fumarate, in combination with other antiretrovirals.
Efavirenz, emtricitabine, and tenofovir disoproxil fumarate is not approved for the treatment of chronic hepatitis B virus (HBV) infection and the safety and efficacy of efavirenz, emtricitabine, and tenofovir disoproxil fumarate have not been established in patients coinfected with HBV and HIV-1. Severe acute exacerbations of hepatitis B have been reported in patients who have discontinued emcitrabine or tenofovir disoproxil fumarate, which are components of efavirenz, emtricitabine, and tenofovir disoproxil fumarate. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who are coinfected with HIV-1 and HBV and discontinue efavirenz, emtricitabine, and tenofovir disoproxil fumarate. If appropriate, initiation of anti-hepatitis B therapy may be warranted.
|
Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs including tenofovir DF, a component of efavirenz, emtricitabine and tenofovir disoproxil fumarate, in combination with other antiretrovirals. A majority of these cases have been in women. Obesity and prolonged nucleoside exposure may be risk factors. Particular caution should be exercised when administering nucleoside analogs to any patient with known risk factors for liver disease; however, cases have also been reported in patients with no known risk factors. Treatment with efavirenz, emtricitabine and tenofovir disoproxil fumarate should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
Patients Coinfected with HIV-1 and HBV
It is recommended that all patients with HIV-1 be tested for the presence of chronic HBV before initiating antiretroviral therapy. Efavirenz, emtricitabine and tenofovir disoproxil fumarate is not approved for the treatment of chronic HBV infection, and the safety and efficacy of efavirenz, emtricitabine and tenofovir disoproxil fumarate have not been established in patients coinfected with HBV and HIV-1. Severe acute exacerbations of hepatitis B have been reported in patients who are coinfected with HBV and HIV-1 and have discontinued emtricitabine or tenofovir DF, two of the components of efavirenz, emtricitabine and tenofovir disoproxil fumarate. In some patients infected with HBV and treated with emtricitabine, the exacerbations of hepatitis B were associated with liver decompensation and liver failure. Patients who are coinfected with HIV-1 and HBV should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment with efavirenz, emtricitabine and tenofovir disoproxil fumarate. If appropriate, initiation of anti-hepatitis B therapy may be warranted.
Efavirenz, emtricitabine and tenofovir disoproxil fumarate should not be administered with adefovir dipivoxil.
Drug Interactions
Efavirenz plasma concentrations may be altered by substrates, inhibitors, or inducers of CYP3A. Likewise, efavirenz may alter plasma concentrations of drugs metabolized by CYP3A or CYP2B6.
Coadministration with Related Products
Related drugs not for coadministration with efavirenz, emtricitabine and tenofovir disoproxil fumarate include emtricitabine/rilpivirine/tenofovir DF, emtricitabine, elvitegravir/cobicistat/emtricitabine/tenofovir DF, emtricitabine/tenofovir DF, and tenofovir DF, which contain the same active components as efavirenz, emtricitabine and tenofovir disoproxil fumarate. Efavirenz should not be coadministered with efavirenz, emtricitabine and tenofovir disoproxil fumarate unless needed for dose-adjustment (e.g., with rifampin). Due to similarities between emtricitabine and lamivudine, efavirenz, emtricitabine and tenofovir disoproxil fumarate should not be coadministered with drugs containing lamivudine, including lamivudine/zidovudine, lamivudine, abacavir sulfate/lamivudine), or abacavir sulfate/lamivudine/zidovudine.
Psychiatric Symptoms
Serious psychiatric adverse experiences have been reported in patients treated with efavirenz. In controlled trials of 1008 subjects treated with regimens containing efavirenz for a mean of 2.1 years and 635 subjects treated with control regimens for a mean of 1.5 years, the frequency (regardless of causality) of specific serious psychiatric events among subjects who received efavirenz or control regimens, respectively, were: severe depression (2.4%, 0.9%), suicidal ideation (0.7%, 0.3%), nonfatal suicide attempts (0.5%, 0%), aggressive behavior (0.4%, 0.5%), paranoid reactions (0.4%, 0.3%), and manic reactions (0.2%, 0.3%). When psychiatric symptoms similar to those noted above were combined and evaluated as a group in a multifactorial analysis of data from Study AI266006 (006), treatment with efavirenz was associated with an increase in the occurrence of these selected psychiatric symptoms. Other factors associated with an increase in the occurrence of these psychiatric symptoms were history of injection drug use, psychiatric history, and receipt of psychiatric medication at trial entry; similar associations were observed in both the efavirenz and control treatment groups. In Study 006, onset of new serious psychiatric symptoms occurred throughout the trial for both efavirenz-treated and control-treated subjects. One percent of efavirenz-treated subjects discontinued or interrupted treatment because of one or more of these selected psychiatric symptoms. There have also been occasional postmarketing reports of death by suicide, delusions, and psychosis-like behavior, although a causal relationship to the use of efavirenz cannot be determined from these reports. Patients with serious psychiatric adverse experiences should seek immediate medical evaluation to assess the possibility that the symptoms may be related to the use of efavirenz, and if so, to determine whether the risks of continued therapy outweigh the benefits.
Nervous System Symptoms
Fifty-three percent (531/1008) of subjects receiving efavirenz in controlled trials reported central nervous system symptoms (any grade, regardless of causality) compared to 25% (156/635) of subjects receiving control regimens. These symptoms included dizziness (28.1% of the 1008 subjects), insomnia (16.3%), impaired concentration (8.3%), somnolence (7.0%), abnormal dreams (6.2%), and hallucinations (1.2%). Other reported symptoms were euphoria, confusion, agitation, amnesia, stupor, abnormal thinking, and depersonalization. The majority of these symptoms were mild-to-moderate (50.7%); symptoms were severe in 2.0% of subjects. Overall, 2.1% of subjects discontinued therapy as a result. These symptoms usually begin during the first or second day of therapy and generally resolve after the first 2–4 weeks of therapy. After 4 weeks of therapy, the prevalence of nervous system symptoms of at least moderate severity ranged from 5% to 9% in subjects treated with regimens containing efavirenz and from 3% to 5% in subjects treated with a control regimen. Patients should be informed that these common symptoms were likely to improve with continued therapy and were not predictive of subsequent onset of the less frequent psychiatric symptom. Dosing at bedtime may improve the tolerability of these nervous system symptoms.
Analysis of long-term data from Study 006 (median follow-up 180 weeks, 102 weeks, and 76 weeks for subjects treated with efavirenz + zidovudine + lamivudine, efavirenz + indinavir, and indinavir + zidovudine + lamivudine, respectively) showed that, beyond 24 weeks of therapy, the incidences of new-onset nervous system symptoms among efavirenz-treated subjects were generally similar to those in the indinavir-containing control arm.
Patients receiving efavirenz, emtricitabine and tenofovir disoproxil fumarate should be alerted to the potential for additive central nervous system effects when efavirenz, emtricitabine and tenofovir disoproxil fumarate is used concomitantly with alcohol or psychoactive drugs.
Patients who experience central nervous system symptoms such as dizziness, impaired concentration, and/or drowsiness should avoid potentially hazardous tasks such as driving or operating machinery.
New Onset or Worsening Renal Impairment
Emtricitabine and tenofovir are principally eliminated by the kidney; however, efavirenz is not. Since efavirenz, emtricitabine and tenofovir disoproxil fumarate is a combination product and the dose of the individual components cannot be altered, patients with estimated creatinine clearance below 50 mL/min should not receive efavirenz, emtricitabine and tenofovir disoproxil fumarate.
Renal impairment, including cases of acute renal failure and Fanconi syndrome (renal tubular injury with severe hypophosphatemia), has been reported with the use of tenofovir DF.
It is recommended that estimated creatinine clearance be assessed in all patients prior to initiating therapy and as clinically appropriate during therapy with efavirenz, emtricitabine and tenofovir disoproxil fumarate. In patients at risk of renal dysfunction, including patients who have previously experienced renal events while receiving adefovir dipivoxil, it is recommended that estimated creatinine clearance, serum phosphorus, urine glucose, and urine protein be assessed prior to initiation of efavirenz, emtricitabine and tenofovir disoproxil fumarate, and periodically during efavirenz, emtricitabine and tenofovir disoproxil fumarate therapy.
efavirenz, emtricitabine and tenofovir disoproxil fumarate should be avoided with concurrent or recent use of a nephrotoxic agent (e.g., high-dose or multiple non-steroidal anti-inflammatory drugs (NSAIDs)). Cases of acute renal failure after initiation of high dose or multiple NSAIDs have been reported in HIV-infected patients with risk factors for renal dysfunction who appeared stable on tenofovir DF. Some patients required hospitalization and renal replacement therapy. Alternatives to NSAIDs should be considered, if needed, in patients at risk for renal dysfunction.
Persistent or worsening bone pain, pain in extremities, fractures and/or muscular pain or weakness may be manifestations of proximal renal tubulopathy and should prompt an evaluation of renal function in at-risk patients.
Reproductive Risk Potential
Pregnancy Category D: Efavirenz may cause fetal harm when administered during the first trimester to a pregnant woman. Pregnancy should be avoided in women receiving efavirenz, emtricitabine and tenofovir disoproxil fumarate. Barrier contraception must always be used in combination with other methods of contraception (e.g., oral or other hormonal contraceptives). Because of the long half-life of efavirenz, use of adequate contraceptive measures for 12 weeks after discontinuation of efavirenz, emtricitabine and tenofovir disoproxil fumarate is recommended. Women of childbearing potential should undergo pregnancy testing before initiation of efavirenz, emtricitabine and tenofovir disoproxil fumarate. If this drug is used during the first trimester of pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential harm to the fetus.
There are no adequate and well-controlled trials of efavirenz, emtricitabine and tenofovir disoproxil fumarate in pregnant women. Efavirenz, emtricitabine and tenofovir disoproxil fumarate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus, such as in pregnant women without other therapeutic options.
Rash
In controlled clinical trials, 26% (266/1008) of subjects treated with 600 mg efavirenz experienced new-onset skin rash compared with 17% (111/635) of subjects treated in control groups. Rash associated with blistering, moist desquamation, or ulceration occurred in 0.9% (9/1008) of subjects treated with efavirenz. The incidence of Grade 4 rash (e.g., erythema multiforme, Stevens-Johnson syndrome) in subjects treated with efavirenz in all trials and expanded access was 0.1%. Rashes are usually mild-to-moderate maculopapular skin eruptions that occur within the first 2 weeks of initiating therapy with efavirenz (median time to onset of rash in adults was 11 days) and, in most subjects continuing therapy with efavirenz, rash resolves within 1 month (median duration, 16 days). The discontinuation rate for rash in clinical trials was 1.7% (17/1008). efavirenz, emtricitabine and tenofovir disoproxil fumarate can be reinitiated in patients interrupting therapy because of rash. Efavirenz, emtricitabine and tenofovir disoproxil fumarate should be discontinued in patients developing severe rash associated with blistering, desquamation, mucosal involvement, or fever. Appropriate antihistamines and/or corticosteroids may improve the tolerability and hasten the resolution of rash. For patients who have had a life-threatening cutaneous reaction (e.g., Stevens-Johnson syndrome), alternative therapy should be considered.
Experience with efavirenz in subjects who discontinued other antiretroviral agents of the NNRTI class is limited. Nineteen subjects who discontinued nevirapine because of rash have been treated with efavirenz. Nine of these subjects developed mild-to-moderate rash while receiving therapy with efavirenz, and two of these subjects discontinued because of rash.
Rash was reported in 26 of 57 pediatric subjects (46%) treated with efavirenz. One pediatric subject experienced Grade 3 rash (confluent rash with fever), and two subjects had Grade 4 rash (erythema multiforme). The median time to onset of rash in pediatric subjects was 8 days. Prophylaxis with appropriate antihistamines before initiating therapy with efavirenz, emtricitabine and tenofovir disoproxil fumarate in pediatric patients should be considered.
Hepatotoxicity
Monitoring of liver enzymes before and during treatment is recommended for patients with underlying hepatic disease, including hepatitis B or hepatitis C infection; patients with marked transaminase elevations; and patients treated with other medications associated with liver toxicity. A few of the postmarketing reports of hepatic failure occurred in patients with no pre-existing hepatic disease or other identifiable risk factors. Liver enzyme monitoring should also be considered for patients without pre-existing hepatic dysfunction or other risk factors. In patients with persistent elevations of serum transaminases to greater than five times the upper limit of the normal range, the benefit of continued therapy with efavirenz, emtricitabine and tenofovir disoproxil fumarate needs to be weighed against the unknown risks of significant liver toxicity.
Bone Effects of Tenofovir DF
Bone Mineral Density
In clinical trials in HIV-1 infected adults, tenofovir DF was associated with slightly greater decreases in bone mineral density (BMD) and increases in biochemical markers of bone metabolism, suggesting increased bone turnover relative to comparators. Serum parathyroid hormone levels and 1,25 Vitamin D levels were also higher in subjects receiving tenofovir DF.
Clinical trials evaluating tenofovir DF in pediatric and adolescent subjects were conducted. Under normal circumstances, BMD increases rapidly in pediatric patients. In HIV-1 infected subjects aged 2 years to less than 18 years, bone effects were similar to those observed in adult subjects and suggest increased bone turnover. Total body BMD gain was less in the tenofovir DF treated HIV-1 infected pediatric subjects as compared to the control groups. Similar trends were observed in chronic hepatitis B infected adolescent subjects aged 12 years to less than 18 years. In all pediatric trials, skeletal growth (height) appeared to be unaffected. For more information, consult the tenofovir disoproxil fumarate prescribing information.
The effects of tenofovir DF-associated changes in BMD and biochemical markers on long-term bone health and future fracture risk are unknown. Assessment of BMD should be considered for adult and pediatric patients who have a history of pathologic bone fracture or other risk factors for osteoporosis or bone loss. Although the effect of supplementation with calcium and vitamin D was not studied, such supplementation may be beneficial for all patients. If bone abnormalities are suspected then appropriate consultation should be obtained.
Mineralization Defects
Cases of osteomalacia associated with proximal renal tubulopathy, manifested as bone pain or pain in extremities and which may contribute to fractures, have been reported in association with the use of tenofovir DF. Arthralgias and muscle pain or weakness have also been reported in cases of proximal renal tubulopathy. Hypophosphatemia and osteomalacia secondary to proximal renal tubulopathy should be considered in patients at risk of renal dysfunction who present with persistent or worsening bone or muscle symptoms while receiving products containing tenofovir DF.
Convulsions
Convulsions have been observed in patients receiving efavirenz, generally in the presence of known medical history of seizures. Caution must be taken in any patient with a history of seizures.
Patients who are receiving concomitant anticonvulsant medications primarily metabolized by the liver, such as phenytoin and phenobarbital, may require periodic monitoring of plasma levels.
Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including the components of efavirenz, emtricitabine and tenofovir disoproxil fumarate. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution, however, the time to onset is more variable, and can occur many months after initiation of treatment.
Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trials in Adult Subjects
Study 934
Study 934 was an open-label active-controlled trial in which 511 antiretroviral-naive subjects received either emtricitabine + tenofovir DF administered in combination with efavirenz (N=257) or zidovudine/lamivudine administered in combination with efavirenz (N=254).
The most common adverse reactions (incidence greater than or equal to 10%, any severity) occurring in Study 934 include diarrhea, nausea, fatigue, headache, dizziness, depression, insomnia, abnormal dreams, and rash. Adverse reactions observed in Study 934 were generally consistent with those seen in previous trials of the individual components (Table 2).
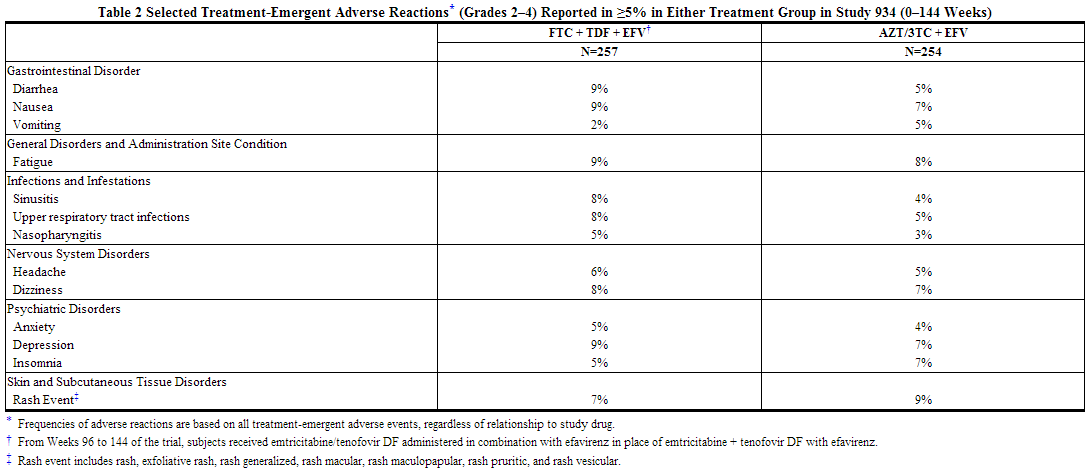
Study 073
In Study 073, subjects with stable, virologic suppression on antiretroviral therapy and no history of virologic failure were randomized to receive efavirenz, emtricitabine and tenofovir disoproxil fumarate or to stay on their baseline regimen. The adverse reactions observed in Study 073 were generally consistent with those seen in Study 934 and those seen with the individual components of efavirenz, emtricitabine and tenofovir disoproxil fumarate when each was administered in combination with other antiretroviral agents.
Efavirenz, Emtricitabine, or Tenofovir Disoproxil Fumarate
In addition to the adverse reactions in Study 934 and Study 073, the following adverse reactions were observed in clinical trials of efavirenz, emtricitabine, or tenofovir DF in combination with other antiretroviral agents.
Efavirenz: The most significant adverse reactions observed in subjects treated with efavirenz are nervous system symptoms, psychiatric symptoms, and rash.
Selected adverse reactions of moderate-to-severe intensity observed in greater than or equal to 2% of efavirenz-treated subjects in two controlled clinical trials included pain, impaired concentration, abnormal dreams, somnolence, anorexia, dyspepsia, abdominal pain, nervousness, and pruritus.
Pancreatitis has also been reported, although a causal relationship with efavirenz has not been established. Asymptomatic increases in serum amylase levels were observed in a significantly higher number of subjects treated with efavirenz 600 mg than in control subjects.
Emtricitabine and Tenofovir Disoproxil Fumarate: Adverse reactions that occurred in at least 5% of treatment-experienced or treatment-naive subjects receiving emtricitabine or tenofovir DF with other antiretroviral agents in clinical trials include arthralgia, increased cough, dyspepsia, fever, myalgia, pain, abdominal pain, back pain, paresthesia, peripheral neuropathy (including peripheral neuritis and neuropathy), pneumonia, rhinitis and rash event (including rash, pruritus, maculopapular rash, urticaria, vesiculobullous rash, pustular rash, and allergic reaction).
Skin discoloration has been reported with higher frequency among emtricitabine-treated subjects; it was manifested by hyperpigmentation on the palms and/or soles and was generally mild and asymptomatic. The mechanism and clinical significance are unknown.
Clinical Trials in Pediatric Subjects
Efavirenz: In a pediatric clinical trial in 57 NRTI-experienced subjects aged 3 to 16 years, the type and frequency of adverse experiences was generally similar to that of adult subjects with the exception of a higher incidence of rash, which was reported in 46% (26/57) of pediatric subjects compared to 26% of adults, and a higher frequency of Grade 3 or 4 rash reported in 5% (3/57) of pediatric subjects compared to 0.9% of adults. For additional information, please consult the efavirenz prescribing information.
Emtricitabine: In addition to the adverse reactions reported in adults, anemia and hyperpigmentation were observed in 7% and 32%, respectively, of pediatric subjects (3 months to less than 18 years of age) who received treatment with emtricitabine in the larger of two open-label, uncontrolled pediatric trials (N=116). For additional information, please consult the emtricitabine prescribing information.
Tenofovir Disoproxil Fumarate: In a pediatric clinical trial conducted in subjects 12 to less than 18 years of age, the adverse reactions observed in pediatric subjects who received treatment with tenofovir DF were consistent with those observed in clinical trials of tenofovir DF in adults.
Laboratory Abnormalities
Efavirenz, emtricitabine and tenofovir disoproxil fumarate: Laboratory abnormalities observed in Study 934 were generally consistent with those seen in previous trials (Table 3).
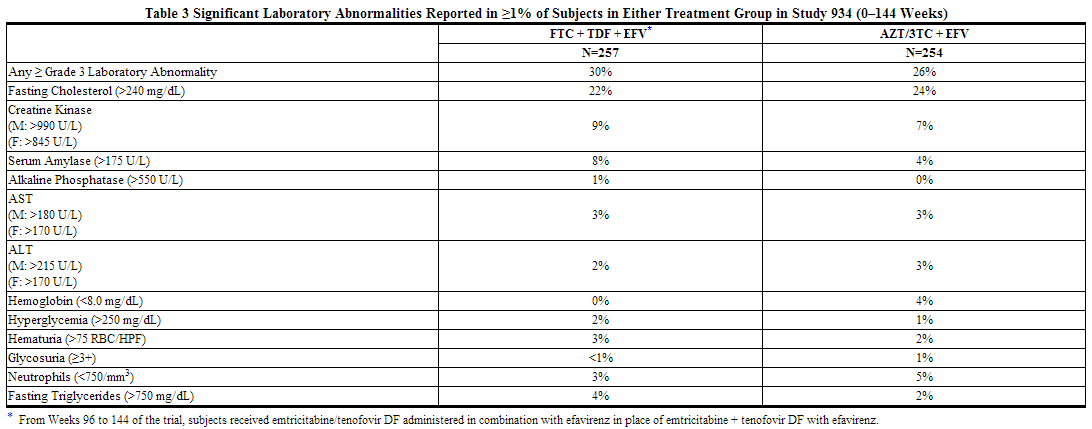
Laboratory abnormalities observed in Study 073 were generally consistent with those in Study 934.
In addition to the laboratory abnormalities described for Study 934 (Table 3), Grade 3/4 laboratory abnormalities of increased bilirubin (greater than 2.5 × upper limit of normal (ULN)), increased pancreatic amylase (greater than 2.0 × ULN), increased or decreased serum glucose (less than 40 or greater than 250 mg/dL), and increased serum lipase (greater than 2.0 × ULN) occurred in up to 3% of subjects treated with emtricitabine or tenofovir DF with other antiretroviral agents in clinical trials.
Hepatic Events: In Study 934, 19 subjects treated with efavirenz, emtricitabine, and tenofovir DF and 20 subjects treated with efavirenz and fixed-dose zidovudine/lamivudine were hepatitis B surface antigen or hepatitis C antibody positive. Among these coinfected subjects, one subject (1/19) in the efavirenz, emtricitabine and tenofovir DF arm had elevations in transaminases to greater than five times ULN through 144 weeks. In the fixed-dose zidovudine/lamivudine arm, two subjects (2/20) had elevations in transaminases to greater than five times ULN through 144 weeks. No HBV and/or HCV coinfected subject discontinued from the trial due to hepatobiliary disorders.
Postmarketing Experience
The following adverse reactions have been identified during postapproval use of efavirenz, emtricitabine, or tenofovir DF. Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Efavirenz:
- Cardiac Disorders: Palpitations
- Ear and Labyrinth Disorders: Tinnitus, vertigo
- Endocrine Disorders: Gynecomastia
- Eye Disorders: Abnormal vision
- Gastrointestinal Disorders: Constipation, malabsorption
- General Disorders and Administration Site Conditions: Asthenia
- Hepatobiliary Disorders: Hepatic enzyme increase, hepatic failure, hepatitis. A few of the postmarketing reports of hepatic failure, including cases in patients with no pre-existing hepatic disease or other identifiable risk factors, were characterized by a fulminant course, progressing in some cases to transplantation or death.
- Immune System Disorders: Allergic reactions
- Metabolism and Nutrition Disorders: Redistribution/accumulation of body fat, hypercholesterolemia, hypertriglyceridemia
- Musculoskeletal and Connective Tissue Disorders: Arthralgia, myalgia, myopathy
- Nervous System Disorders: Abnormal coordination, ataxia, cerebellar coordination and balance disturbances, convulsions, hypoesthesia, paresthesia, neuropathy, tremor
- Psychiatric Disorders: Aggressive reactions, agitation, delusions, emotional lability, mania, neurosis, paranoia, psychosis, suicide
- Respiratory, Thoracic and Mediastinal Disorders: Dyspnea
- Skin and Subcutaneous Tissue Disorders: Flushing, erythema multiforme, photoallergic dermatitis, Stevens-Johnson syndrome
Emtricitabine: No postmarketing adverse reactions have been identified for inclusion in this section.
Tenofovir Disoproxil Fumarate:
- Immune System Disorders: Allergic reaction, including angioedema
- Metabolism and Nutrition Disorders: Lactic acidosis, hypokalemia, hypophosphatemia
- Respiratory, Thoracic, and Mediastinal Disorders: Dyspnea
- Gastrointestinal Disorders: Pancreatitis, increased amylase, abdominal pain
- Hepatobiliary Disorders: Hepatic steatosis, hepatitis, increased liver enzymes (most commonly AST, ALT, GGT)
- Skin and Subcutaneous Tissue Disorders: Rash
- Musculoskeletal and Connective Tissue Disorders: Rhabdomyolysis, osteomalacia (manifested as bone pain and which may contribute to fractures), muscular weakness, myopathy
- Renal and Urinary Disorders: Acute renal failure, renal failure, acute tubular necrosis, Fanconi syndrome, proximal renal tubulopathy, interstitial nephritis (including acute cases), nephrogenic diabetes insipidus, renal insufficiency, increased creatinine, proteinuria, polyuria
- General Disorders and Administration Site Conditions: Asthenia
The following adverse reactions, listed under the body system headings above, may occur as a consequence of proximal renal tubulopathy: rhabdomyolysis, osteomalacia, hypokalemia, muscular weakness, myopathy, hypophosphatemia.
Drug Interactions
This section describes clinically relevant drug interactions with efavirenz, emtricitabine and tenofovir disoproxil fumarat. Drug interaction trials are described elsewhere in the labeling.
=Efavirenz
Efavirenz has been shown in vivo to induce CYP3A and CYP2B6. Other compounds that are substrates of CYP3A or CYP2B6 may have decreased plasma concentrations when coadministered with efavirenz. In vitro studies have demonstrated that efavirenz inhibits CYP2C9, 2C19, and 3A4 isozymes in the range of observed efavirenz plasma concentrations. Coadministration of efavirenz with drugs primarily metabolized by these isozymes may result in altered plasma concentrations of the coadministered drug. Therefore, appropriate dose adjustments may be necessary for these drugs.
Drugs that induce CYP3A activity (e.g., phenobarbital, rifampin, rifabutin) would be expected to increase the clearance of efavirenz, resulting in lowered plasma concentrations.
Emtricitabine and Tenofovir Disoproxil Fumarate
Since emtricitabine and tenofovir are primarily eliminated by the kidneys, coadministration of efavirenz, emtricitabine and tenofovir disoproxil fumarate with drugs that reduce renal function or compete for active tubular secretion may increase serum concentrations of emtricitabine, tenofovir, and/or other renally eliminated drugs. Some examples include, but are not limited to, acyclovir, adefovir dipivoxil, cidofovir, ganciclovir, valacyclovir, valganciclovir, aminoglycosides (e.g., gentamicin), and high-dose or multiple NSAIDs.
Coadministration of tenofovir DF and didanosine should be undertaken with caution and patients receiving this combination should be monitored closely for didanosine-associated adverse reactions. Didanosine should be discontinued in patients who develop didanosine-associated adverse reactions. Suppression of CD4+ cell counts has been observed in patients receiving tenofovir DF with didanosine 400 mg daily.
Darunavir with ritonavir and lopinavir/ritonavir have been shown to increase tenofovir concentrations. Tenofovir DF is a substrate of P-glycoprotein (Pgp) and breast cancer resistance protein (BCRP) transporters. When tenofovir DF is co-administered with an inhibitor of these transporters, an increase in absorption may be observed. Patients receiving darunavir with ritonavir and efavirenz, emtricitabine and tenofovir disoproxil fumarate, or lopinavir/ritonavir with efavirenz, emtricitabine and tenofovir disoproxil fumarate, should be monitored for tenofovir-associated adverse reactions. Efavirenz, emtricitabine and tenofovir disoproxil fumarate should be discontinued in patients who develop tenofovir-associated adverse reactions.
Coadministration of atazanavir with efavirenz, emtricitabine and tenofovir disoproxil fumarate is not recommended since coadministration of atazanavir with either efavirenz or tenofovir DF has been shown to decrease plasma concentrations of atazanavir. Also, atazanavir has been shown to increase tenofovir concentrations. There are insufficient data to support dosing recommendations for atazanavir or atazanavir/ritonavir in combination with efavirenz, emtricitabine and tenofovir disoproxil fumarate.
Efavirenz, Emtricitabine and Tenofovir Disoproxil Fumarate
Other important drug interaction information for efavirenz, emtricitabine and tenofovir disoproxil fumarate is summarized in Table 1 and Table 4. The drug interactions described are based on trials conducted with efavirenz, emtricitabine or tenofovir DF as individual agents or are potential drug interactions; no drug interaction trials have been conducted using efavirenz, emtricitabine and tenofovir disoproxil fumarate. The tables include potentially significant interactions, but are not all inclusive.
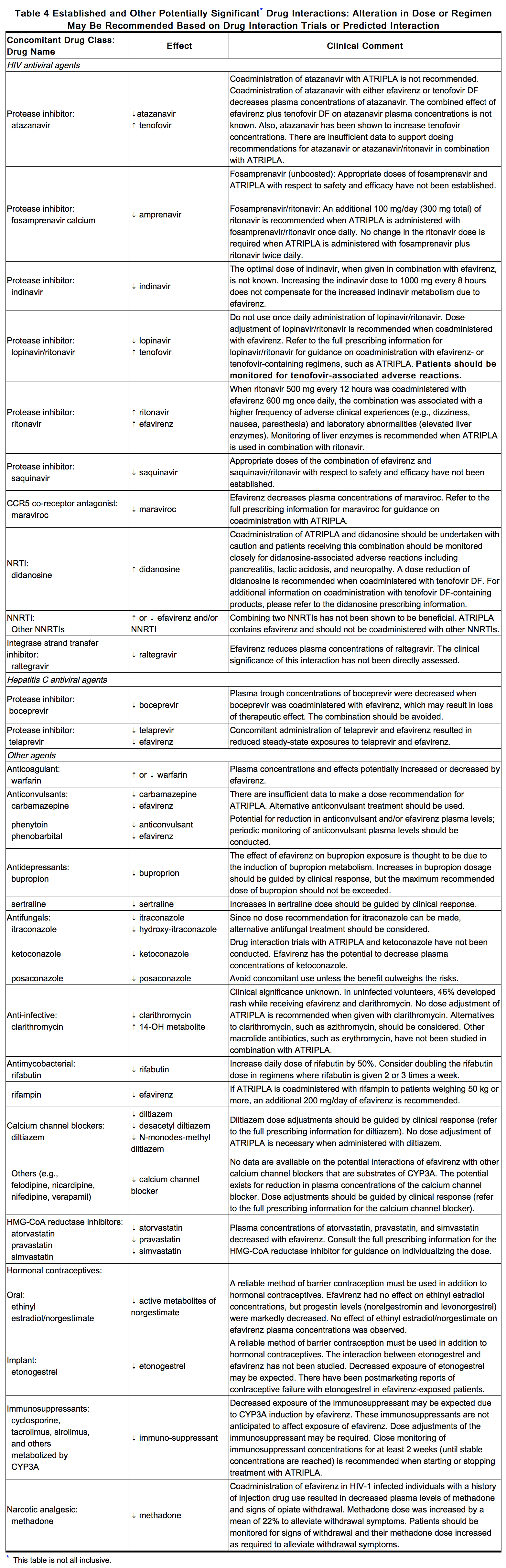
Efavirenz Assay Interference
Cannabinoid Test Interaction: Efavirenz does not bind to cannabinoid receptors. False-positive urine cannabinoid test results have been observed in non-HIV-infected volunteers receiving efavirenz when the Microgenics Cedia DAU Multi-Level THC assay was used for screening. Negative results were obtained when more specific confirmatory testing was performed with gas chromatography/mass spectrometry. For more information, please consult the efavirenz prescribing information.
Use in Specific Populations
Pregnancy
Antiretroviral Pregnancy Registry
To monitor fetal outcomes of pregnant women, an Antiretroviral Pregnancy Registry has been established. Physicians are encouraged to register patients who become pregnant by calling (800) 258-4263.
Efavirenz: As of July 2010, the Antiretroviral Pregnancy Registry has received prospective reports of 792 pregnancies exposed to efavirenz-containing regimens, nearly all of which were first-trimester exposures (718 pregnancies). Birth defects occurred in 17 of 604 live births (first-trimester exposure) and 2 of 69 live births (second/third-trimester exposure). One of these prospectively reported defects with first-trimester exposure was a neural tube defect. A single case of anophthalmia with first-trimester exposure to efavirenz has also been prospectively reported; however, this case included severe oblique facial clefts and amniotic banding, a known association with anophthalmia. There have been six retrospective reports of findings consistent with neural tube defects, including meningomyelocele. All mothers were exposed to efavirenz-containing regimens in the first trimester. Although a causal relationship of these events to the use of efavirenz has not been established, similar defects have been observed in preclinical studies of efavirenz.
Animal Data
Effects of efavirenz on embryo-fetal development have been studied in three nonclinical species (cynomolgus monkeys, rats, and rabbits). In monkeys, efavirenz 60 mg/kg/day was administered to pregnant females throughout pregnancy (gestation Days 20 through 150). The maternal systemic drug exposures (AUC) were 1.3 times the exposure in humans at the recommended clinical dose (600 mg/day), with fetal umbilical venous drug concentrations approximately 0.7 times the maternal values. Three fetuses of 20 fetuses/infants had one or more malformations; there were no malformed fetuses or infants from placebo-treated mothers. The malformations that occurred in these three monkey fetuses included anencephaly and unilateral anophthalmia in one fetus, microophthalmia in a second, and cleft palate in the third. There was no NOAEL (no observable adverse effect level) established for this study because only one dosage was evaluated. In rats, efavirenz was administered either during organogenesis (gestation Days 7 to 18) or from gestation Day 7 through lactation Day 21 at 50, 100, or 200 mg/kg/day. Administration of 200 mg/kg/day in rats was associated with an increase in the incidence of early resorptions, and doses 100 mg/kg/day and greater were associated with early neonatal mortality. The AUC at the NOAEL (50 mg/kg/day) in this rat study was 0.1 times that in humans at the recommended clinical dose. Drug concentrations in the milk on lactation Day 10 were approximately 8 times higher than those in maternal plasma. In pregnant rabbits, efavirenz was neither embryo lethal nor teratogenic when administered at doses of 25, 50, and 75 mg/kg/day over the period of organogenesis (gestation Days 6 through 18). The AUC at the NOAEL (75 mg/kg/day) in rabbits was 0.4 times that in humans at the recommended clinical dose.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Efavirenz, emtricitabine and tenofovir disoproxil fumarate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Efavirenz, emtricitabine and tenofovir disoproxil fumarate during labor and delivery.
Nursing Mothers
The Centers for Disease Control and Prevention recommend that HIV-1 infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV-1. Studies in rats have demonstrated that efavirenz is secreted in milk. Studies in humans have shown that both tenofovir and emtricitabine are excreted in human milk. Because the risks of low level exposure to emtricitabine and tenofovir to infants are unknown, and because of the potential for HIV-1 transmission, mothers should be instructed not to breastfeed if they are receiving efavirenz, emtricitabine and tenofovir disoproxil fumarate.
Emtricitabine
Samples of breast milk obtained from five HIV-1 infected mothers show that emtricitabine is secreted in human milk. Breastfeeding infants whose mothers are being treated with emtricitabine may be at risk for developing viral resistance to emtricitabine. Other emtricitabine-associated risks in infants breastfed by mothers being treated with emtricitabine are unknown.
Tenofovir Disoproxil Fumarate
Samples of breast milk obtained from five HIV-1 infected mothers show that tenofovir is secreted in human milk. Tenofovir-associated risks, including the risk of viral resistance to tenofovir, in infants breastfed by mothers being treated with tenofovir disoproxil fumarate are unknown.
Pediatric Use
Efavirenz, emtricitabine and tenofovir disoproxil fumarate should only be administered to pediatric patients 12 years of age and older with a body weight greater than or equal to 40 kg (greater than or equal to 88 lbs). Because efavirenz, emtricitabine and tenofovir disoproxil fumarateis a fixed-dose combination tablet, the dose adjustments recommended for pediatric patients younger than 12 years of age for each individual component cannot be made with efavirenz, emtricitabine and tenofovir disoproxil fumarate.
Geriatic Use
Clinical trials of efavirenz, emtricitabine and tenofovir DF did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for elderly patients should be cautious, keeping in mind the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Efavirenz, emtricitabine and tenofovir disoproxil fumarate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Efavirenz, emtricitabine and tenofovir disoproxil fumarate with respect to specific racial populations.
Renal Impairment
Because efavirenz, emtricitabine and tenofovir DF is a fixed-dose combination, it should not be prescribed for patients requiring dosage adjustment such as those with moderate or severe renal impairment (estimated creatinine clearance below 50 mL/min).
Hepatic Impairment
Efavirenz, emtricitabine and tenofovir disoproxil fumarate is not recommended for patients with moderate or severe hepatic impairment because there are insufficient data to determine an appropriate dose. Patients with mild hepatic impairment may be treated with efavirenz, emtricitabine and tenofovir disoproxil fumarate at the approved dose. Because of the extensive cytochrome P450-mediated metabolism of efavirenz and limited clinical experience in patients with hepatic impairment, caution should be exercised in administering efavirenz, emtricitabine and tenofovir disoproxil fumarate to these patients.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Efavirenz, emtricitabine and tenofovir disoproxil fumarate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Efavirenz, emtricitabine and tenofovir disoproxil fumarate in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
HVB
Efavirenz, emtricitabine, and tenofovir disoproxil fumarate tablet is not approved for the treatment of chronic hepatitis B virus (HBV) infection and the safety and efficacy of efavirenz, emtricitabine, and tenofovir disoproxil fumarate have not been established in patients coinfected with HBV and HIV-1. Severe acute exacerbations of hepatitis B have been reported in patients who have discontinued emcitrabine or tenofovir disoproxil fumarate, which are components of efavirenz, emtricitabine, and tenofovir disoproxil fumarate. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who are coinfected with HIV-1 and HBV and discontinue efavirenz, emtricitabine, and tenofovir disoproxil fumarate. If appropriate, initiation of anti-hepatitis B therapy may be warranted.
IV Compatibility
There is limited information regarding the compatibility of Efavirenz, emtricitabine and tenofovir disoproxil fumarate and IV administrations.
Overdosage
If overdose occurs, the patient should be monitored for evidence of toxicity, including monitoring of vital signs and observation of the patient's clinical status; standard supportive treatment should then be applied as necessary. Administration of activated charcoal may be used to aid removal of unabsorbed efavirenz. Hemodialysis can remove both emtricitabine and tenofovir DF (refer to detailed information below), but is unlikely to significantly remove efavirenz from the blood.
Efavirenz
Some patients accidentally taking 600 mg twice daily have reported increased nervous system symptoms. One patient experienced involuntary muscle contractions.
Emtricitabine
Limited clinical experience is available at doses higher than the therapeutic dose of emtricitabine. In one clinical pharmacology trial single doses of emtricitabine 1200 mg were administered to 11 subjects. No severe adverse reactions were reported.
Hemodialysis treatment removes approximately 30% of the emtricitabine dose over a 3-hour dialysis period starting within 1.5 hours of emtricitabine dosing (blood flow rate of 400 mL/min and a dialysate flow rate of 600 mL/min). It is not known whether emtricitabine can be removed by peritoneal dialysis.
Tenofovir Disoproxil Fumarate
Limited clinical experience at doses higher than the therapeutic dose of tenofovir DF 300 mg is available. In one trial, 600 mg tenofovir DF was administered to 8 subjects orally for 28 days, and no severe adverse reactions were reported. The effects of higher doses are not known.
Tenofovir is efficiently removed by hemodialysis with an extraction coefficient of approximately 54%. Following a single 300 mg dose of tenofovir DF, a 4-hour hemodialysis session removed approximately 10% of the administered tenofovir dose.
Pharmacology
Efavirenz, emtricitabine and tenofovir disoproxil fumarate
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
ATRIPLA is a fixed-dose combination tablet containing efavirenz, emtricitabine, and tenofovir disoproxil fumarate (tenofovir DF). SUSTIVA is the brand name for efavirenz, a non-nucleoside reverse transcriptase inhibitor. EMTRIVA is the brand name for emtricitabine, a synthetic nucleoside analog of cytidine. VIREAD is the brand name for tenofovir DF, which is converted in vivo to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5'-monophosphate. VIREAD and EMTRIVA are the components of TRUVADA.
ATRIPLA tablets are for oral administration. Each tablet contains 600 mg of efavirenz, 200 mg of emtricitabine, and 300 mg of tenofovir DF (which is equivalent to 245 mg of tenofovir disoproxil) as active ingredients. The tablets include the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. The tablets are film-coated with a coating material containing black iron oxide, polyethylene glycol, polyvinyl alcohol, red iron oxide, talc, and titanium dioxide.
Efavirenz: Efavirenz is chemically described as (S)-6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one. Its molecular formula is C14H9ClF3NO2 and its structural formula is:
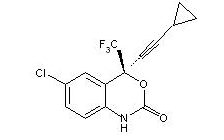
Efavirenz is a white to slightly pink crystalline powder with a molecular mass of 315.68. It is practically insoluble in water (less than 10 µg/mL).
Emtricitabine: The chemical name of emtricitabine is 5-fluoro-1-(2R,5S)-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Emtricitabine is the (-) enantiomer of a thio analog of cytidine, which differs from other cytidine analogs in that it has a fluorine in the 5-position.
It has a molecular formula of C8H10FN3O3S and a molecular weight of 247.24. It has the following structural formula:
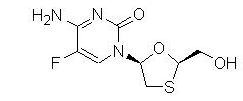
Emtricitabine is a white to off-white crystalline powder with a solubility of approximately 112 mg/mL in water at 25 °C.
Tenofovir Disoproxil Fumarate: Tenofovir DF is a fumaric acid salt of the bis-isopropoxycarbonyloxymethyl ester derivative of tenofovir. The chemical name of tenofovir disoproxil fumarate is 9-[(R)-2([bis([(isopropoxycarbonyl)oxy]- methoxy)phosphinyl]methoxy)propyl]adenine fumarate (1:1). It has a molecular formula of C19H30N5O10P • C4H4O4 and a molecular weight of 635.52. It has the following structural formula:
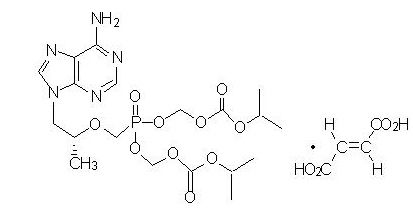
Tenofovir DF is a white to off-white crystalline powder with a solubility of 13.4 mg/mL in water at 25 °C.
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Efavirenz, emtricitabine and tenofovir disoproxil fumarate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Efavirenz, emtricitabine and tenofovir disoproxil fumarate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Efavirenz, emtricitabine and tenofovir disoproxil fumarate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Efavirenz, emtricitabine and tenofovir disoproxil fumarate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Efavirenz, emtricitabine and tenofovir disoproxil fumarate Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.