Cholinesterase enzyme: Difference between revisions
No edit summary |
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
||
| Line 41: | Line 41: | ||
{{CMG}} | {{CMG}} | ||
==Overview== | ==Overview== | ||
Latest revision as of 23:49, 8 August 2012
| acetylcholinesterase (Yt blood group) | |
|---|---|
 | |
| Identifiers | |
| Symbol | ACHE |
| Alt. symbols | YT |
| Entrez | 43 |
| HUGO | 108 |
| OMIM | 100740 |
| RefSeq | NM_015831 |
| UniProt | P22303 |
| Other data | |
| EC number | 3.1.1.7 |
| Locus | Chr. 7 q22 |
| butyrylcholinesterase | |
|---|---|
| Identifiers | |
| Symbol | BCHE |
| Alt. symbols | CHE1 |
| Entrez | 590 |
| HUGO | 983 |
| OMIM | 177400 |
| RefSeq | NM_000055 |
| UniProt | P06276 |
| Other data | |
| EC number | 3.1.1.8 |
| Locus | Chr. 3 q26.1-26.2 |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
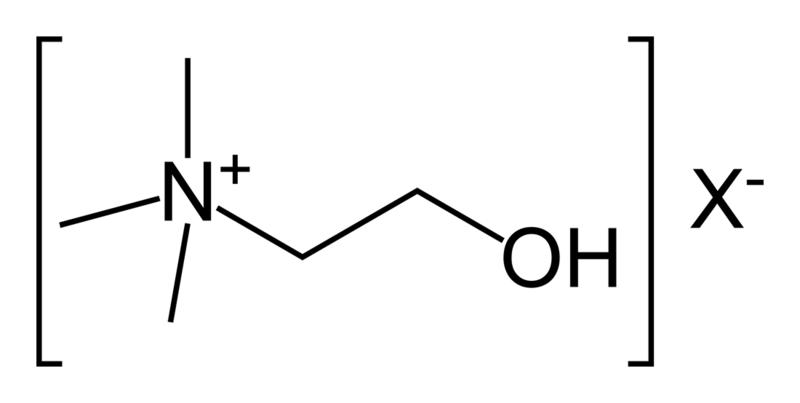
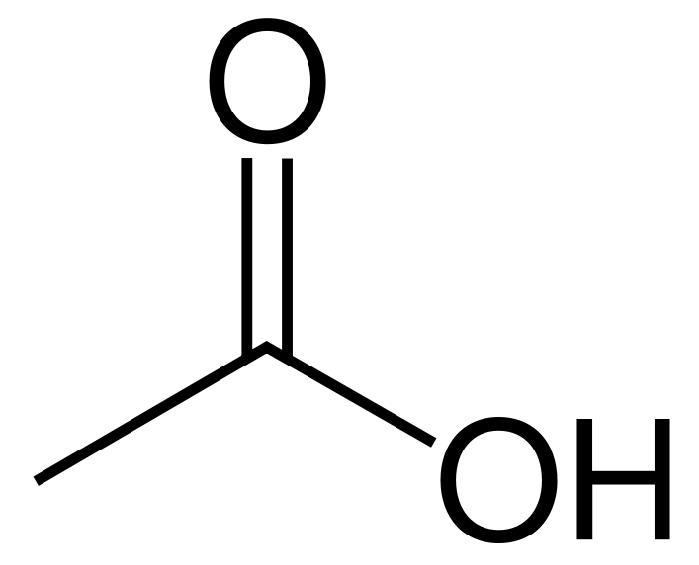
In biochemistry, cholinesterase is an enzyme which catalyzes the hydrolysis of the neurotransmitter acetylcholine into choline and acetic acid, a reaction necessary to allow a cholinergic neuron to return to its resting state after activation.
Types
There are two types:
- Acetylcholinesterase (EC 3.1.1.7) (AChE), also known as RBC cholinesterase, erythrocyte cholinesterase, or (most formally) acetylcholine acetylhydrolase, found primarily in the blood and neural synapses
- Pseudocholinesterase (EC 3.1.1.8) (BChE or BuChE), also known as plasma cholinesterase, butyrylcholinesterase, or (most formally) acylcholine acylhydrolase, found primarily in the liver
The difference between the two types of cholinesterase has to do with their respective preferences for substrates: the former hydrolyses acetylcholine more quickly; the latter hydrolyses butyrylcholine more quickly.
History
In 1968, Walo Leuzinger et al successfully purified and crystallized the enzyme from electric eels at Columbia University, NY. [1][2]
The 3D structure of acetylcholinesterase was first determined in 1991 by Joel Sussman et al using protein from the Pacific electric ray.[3]
Clinically-useful quantities of butyrylcholinesterase were synthesized in 2007 by PharmAthene, through the use of genetically-modified goats.[4]
Clinical significance
An absence or mutation of the pseudocholinesterase enzyme leads to a medical condition known simply as pseudocholinesterase deficiency. This is a silent condition that only manifests itself when people who have the deficiency receive the muscle relaxants succinylcholine or mivacurium during a surgery.
Elevation of plasma pseudocholinesterase was observed in 90.5% cases of acute myocardial infarction.[5]
Acetylcholinesterase is tested in early pregnancy. A sample of amniotic fluid is removed by amniocentesis. The presence of AChE in the amniotic fluid can confirm a neural tube defect, a common type of birth defect.[2]
Butyrylcholinesterase can be used as a prophylactic agent against nerve gas and other organophosphate poisoning.[6]
Cholinesterase inhibitors
A cholinesterase inhibitor (or "anticholinesterase") suppresses the action of the enzyme. Because of its essential function, chemicals that interfere with the action of cholinesterase are potent neurotoxins, causing excessive salivation and eye watering in low doses, followed by muscle spasms and ultimately death (examples are some snake venoms, and the nerve gases sarin and VX). One counteracting medication is pralidoxime.
Among the most common acetylcholinesterase inhibitors are phosphorus-based compounds which are designed to bind to the active site of the enzyme. The structural requirements are a phosphorus atom bearing two lipophilic groups, a leaving group (such as a halide or thiocyanate) and a terminal oxygen. The entry on Lawesson's reagent has some details on one sub-class of the phosphorus-based compounds.
Outside of biochemical warfare, anticholinesterases are also used in anesthesia or in the treatment of myasthenia gravis, glaucoma and Alzheimer's disease. Also, such compounds are used for killing insects in a range of products including sheep dip, organophosphate pesticides, and carbamate pesticides. In addition to acute poisoning as described above, a semi-acute poisoning characterized by strong mental disturbances can occur. Also, prolonged exposure can cause birth defects.
Additional images
Reference
- ↑ * Leuzinger W, Baker AL. Acetylcholinesterase, i. Large-scale purification, homogeneity, and amino acid analysis. Proc Natl Acad Sci U S A. 1967 Feb; 57(2): 446-451. PMCID: 335526
- ↑ Leuzinger W, Baker A L, Cauvin E. Acetylcholinesterase, II. Crystallization, Absorption Spectra, Isoionic Point. Proc Natl Acad Sci U S A, Vol. 59, No. 2 (Feb. 15, 1968), pp. 620-623. PMCID: 224717
- ↑ Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science 1991;253:872-9. PMID 1678899.
- ↑ Nerve gas antidote made by goats
- ↑ Textbook of Medical Biochemistry, MN Chatterjea & Rana Shinde, 6th Ed, 2005 (Pg 565)
- ↑ Nerve gas antidote made by goats
External links
- Movies at weizmann.ac.il showing the structure of acetylcholinesterase and interactions with various inhibitors.
- PDB Molecule of the Month Cholinesterase enzyme
- Acetylcholinesterase at the US National Library of Medicine Medical Subject Headings (MeSH)
- Pseudocholinesterase at the US National Library of Medicine Medical Subject Headings (MeSH)