Flumazenil
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Flumazenil is a benzodiazepine receptor antagonist that is FDA approved for the treatment of reversal of conscious sedation induced with benzodiazepines. Common adverse reactions include diaphoresis, injection site pain, dizziness, headache, abnormal vision, blurred vision, agitation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
General Principles
- Initial: 0.2 mg IV over 15 seconds
- Second dose: 0.2 mg IV may be given and repeated at 60-second intervals as needed (up to a maximum of 4 additional times) to a maximum total dose of 1 mg, if desired level of consciousness is not obtained after waiting 45 seconds.
In High-risk Patients
- Slow the rate of administration of flumazenil
Anesthesia and Conscious Sedation
- 0.2 mg to 1 mg given at 0.2 mg/min
- Resedation may be treated by giving a repeat dose at no less than 20 minute intervals. For repeat treatment, no more than 1 mg (at 0.2 mg/min doses) should be given at any one time and no more than 3 mg should be given in any one hour.
Benzodiazepine Overdose
- 3 mg to 5 mg administered as 0.5 mg/min or 0.2 mg/minute titration rate to slowly awaken the patient over 5 to 10 minutes
Patients Tolerant to Benzodiazepines
- Slower titration rates of 0.1 mg/min and lower total doses may help reduce the frequency of emergent confusion and agitation
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Flumazenil in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Flumazenil in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Flumazenil injection is indicated for the reversal of conscious sedation induced with benzodiazepines
- Dosage
- Patients below the age of 1 year have not been established
- Children 1 year or older, 0.01 mg/kg IV over 15 seconds; if adequate sedation reversal does not occur after an additional 45 seconds, further injections of the same dosage may be repeated at 1-minute intervals, as needed up to 4 times
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Flumazenil in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Flumazenil in pediatric patients.
Contraindications
- In patients with a known hypersensitivity to flumazenil or benzodiazepines.
- In patients who have been given a benzodiazepine for control of a potentially life-threatening condition (e.g., control of intracranial pressure or status epilepticus).
- In patients who are showing signs of serious cyclic antidepressant overdose
Warnings
Risk of Seizures
- The reversal of benzodiazepine effects may be associated with the onset of seizures in certain high-risk populations. Possible risk factors for seizures include: concurrent major sedative-hypnotic drug withdrawal, recent therapy with repeated doses of parenteral benzodiazepines, myoclonic jerking or seizure activity prior to flumazenil administration in overdose cases, or concurrent cyclic antidepressant poisoning.
- Flumazenil is not recommended in cases of serious cyclic antidepressant poisoning, as manifested by motor abnormalities (twitching, rigidity, focal seizure), dysrhythmia (wide QRS, ventricular dysrhythmia, heart block), anticholinergic signs (mydriasis, dry mucosa, hypoperistalsis), and cardiovascular collapse at presentation. In such cases flumazenil should be withheld and the patient should be allowed to remain sedated (with ventilatory and circulatory support as needed) until the signs of antidepressant toxicity have subsided. Treatment with flumazenil has no known benefit to the seriously ill mixed-overdose patient other than reversing sedation and should not be used in cases where seizures (from any cause) are likely.
- Most convulsions associated with flumazenil administration require treatment and have been successfully managed with benzodiazepines, phenytoin or barbiturates. Because of the presence of flumazenil, higher than usual doses of benzodiazepines may be required.
Hypoventilation
- Patients who have received flumazenil for the reversal of benzodiazepine effects (after conscious sedation or general anesthesia) should be monitored for resedation, respiratory depression, or other residual benzodiazepine effects for an appropriate period (up to 120 minutes) based on the dose and duration of effect of the benzodiazepine employed.
- This is because flumazenil has not been established in patients as an effective treatment for hypoventilation due to benzodiazepine administration. In healthy male volunteers, flumazenil is capable of reversing benzodiazepine-induced depression of the ventilatory responses to hypercapnia and hypoxia after a benzodiazepine alone. However, such depression may recur because the ventilatory effects of typical doses of flumazenil (1 mg or less) may wear off before the effects of many benzodiazepines. The effects of flumazenil on ventilatory response following sedation with a benzodiazepine in combination with an opioid are inconsistent and have not been adequately studied. The availability of flumazenil does not diminish the need for prompt detection of hypoventilation and the ability to effectively intervene by establishing an airway and assisting ventilation.
- Overdose cases should always be monitored for resedation until the patients are stable and resedation is unlikely.
Adverse Reactions
Clinical Trials Experience
Serious Adverse Reactions
- Deaths have occurred in patients who received flumazenil in a variety of clinical settings. The majority of deaths occurred in patients with serious underlying disease or in patients who had ingested large amounts of non-benzodiazepine drugs (usually cyclic antidepressants), as part of an overdose.
- Serious adverse events have occurred in all clinical settings, and convulsions are the most common serious adverse events reported. Flumazenil administration has been associated with the onset of convulsions in patients with severe hepatic impairment and in patients who are relying on benzodiazepine effects to control seizures, are physically dependent on benzodiazepines, or who have ingested large doses of other drugs (mixed-drug overdose).
- Two of the 446 patients who received flumazenil in controlled clinical trials for the management of a benzodiazepine overdose had cardiac dysrhythmias (1 ventricular tachycardia, 1 junctional tachycardia).
Adverse reactions by organ system
Body as a Whole
- Fatigue (asthenia, malaise), headache, injection site pain*, injection site reaction (thrombophlebitis, skin abnormality, rash)
Cardiovascular System
- Cutaneous vasodilation (sweating, flushing, hot flushes)
Digestive System
Nervous System
- Agitation (anxiety, nervousness, dry mouth, tremor, palpitations, insomnia, dyspnea, hyperventilation)
- Dizziness (vertigo, ataxia)
- Emotional lability (crying abnormal, depersonalization, euphoria, increased tears, depression, dysphoria, paranoia)
Special Senses
- Abnormal vision (visual field defect, diplopia), paresthesia (sensation abnormal, hypoesthesia)
The following adverse events were observed infrequently (less than 1%) in the clinical studies, but were judged as probably related to flumazenil administration and/or reversal of benzodiazepine effects:
Nervous System
- Confusion (difficulty concentrating, delirium), convulsions, somnolence (stupor)
Special Senses
- Abnormal hearing (transient hearing impairment, hyperacusis, tinnitus)
The following adverse events occurred with frequencies less than 1% in the clinical trials. Their relationship to flumazenil administration is unknown, but they are included as alerting information for the physician.
Body as a Whole
Cardiovascular System
- Arrhythmia (atrial, nodal, ventricular extrasystoles)
- Bradycardia
- Tachycardia
- Hypertension
- Chest pain
Digestive System
Nervous System
- Speech disorder (dysphonia, thick tongue)
Not included in this list is operative site pain that occurred with the same frequency in patients receiving placebo as in patients receiving flumazenil for reversal of sedation following a surgical procedure.
Postmarketing Experience
Nervous System
- Fear, panic attacks in patients with a history of panic disorders.
- Withdrawal symptoms may occur following rapid injection of flumazenil in patients with long-term exposure to benzodiazepines.
Drug Interactions
- Interaction with central nervous system depressants other than benzodiazepines has not been specifically studied; however, no deleterious interactions were seen when flumazenil was administered after narcotics, inhalational anesthetics, muscle relaxants and muscle relaxant antagonists administered in conjunction with sedation or anesthesia.
- Particular caution is necessary when using flumazenil in cases of mixed drug overdosage since the toxic effects (such as convulsions and cardiac dysrhythmias) of other drugs taken in overdose (especially cyclic antidepressants) may emerge with the reversal of the benzodiazepine effect by flumazenil.
- The use of flumazenil is not recommended in epileptic patients who have been receiving benzodiazepine treatment for a prolonged period. Although flumazenil exerts a slight intrinsic anticonvulsant effect, its abrupt suppression of the protective effect of a benzodiazepine agonist can give rise to convulsions in epileptic patients.
- Flumazenil blocks the central effects of benzodiazepines by competitive interaction at the receptor level. The effects of nonbenzodiazepine agonists at benzodiazepine receptors, such as zopiclone, triazolopyridazines and others, are also blocked by flumazenil.
- The pharmacokinetics of benzodiazepines are unaltered in the presence of flumazenil and vice versa.
- There is no pharmacokinetic interaction between ethanol and flumazenil.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C There are no adequate and well-controlled studies of the use of flumazenil in pregnant women. Flumazenil should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Teratogenic Effects
Flumazenil has been studied for teratogenicity in rats and rabbits following oral treatments of up to 150 mg/kg/day. The treatments during the major organogenesis were on days 6 to 15 of gestation in the rat and days 6 to 18 of gestation in the rabbit. No teratogenic effects were observed in rats or rabbits at 150 mg/kg; the dose, based on the available data on the area under the plasma concentration-time curve (AUC) represented 120 times to 600 times the human exposure from a maximum recommended intravenous dose of 5 mg in humans. In rabbits, embryocidal effects (as evidenced by increased preimplantation and postimplantation losses) were observed at 50 mg/kg or 200 times the human exposure from a maximum recommended intravenous dose of 5 mg. The no-effect dose of 15 mg/kg in rabbits represents 60 times the human exposure.
Nonteratogenic Effects
An animal reproduction study was conducted in rats at oral dosages of 5, 25, and 125 mg/kg/day of flumazenil. Pup survival was decreased during the lactating period, pup liver weight at weaning was increased for the high-dose group (125 mg/kg/day) and incisor eruption and ear opening in the offspring were delayed; the delay in ear opening was associated with a delay in the appearance of the auditory startle response. No treatment-related adverse effects were noted for the other dose groups. Based on the available data from AUC, the effect level (125 mg/kg) represents 120 times the human exposure from 5 mg, the maximum recommended intravenous dose in humans. The no-effect level represents 24 times the human exposure from an intravenous dose of 5 mg.
Pregnancy Category (AUS): B3
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Flumazenil in women who are pregnant.
Labor and Delivery
The use of flumazenil to reverse the effects of benzodiazepines used during labor and delivery is not recommended because the effects of the drug in the newborn are unknown.
Nursing Mothers
Caution should be exercised when deciding to administer flumazenil to a nursing woman because it is not known whether flumazenil is excreted in human milk.
Pediatric Use
The pharmacokinetics of flumazenil have been evaluated in 29 pediatric patients ranging in age from 1 to 17 years who had undergone minor surgical procedures. The average doses administered were 0.53 mg (0.044 mg/kg) in patients aged 1 to 5 years, 0.63 mg (0.020 mg/kg) in patients aged 6 to 12 years, and 0.8 mg (0.014 mg/kg) in patients aged 13 to 17 years. Compared to adults, the elimination half-life in pediatric patients was more variable, averaging 40 minutes (range: 20 to 75 minutes). Clearance and volume of distribution, normalized for body weight, were in the same range as those seen in adults, although more variability was seen in the pediatric patients.
Geriatic Use
The pharmacokinetics of flumazenil have been studied in the elderly and are not significantly different from younger patients. Several studies of flumazenil in subjects over the age of 65 and one study in subjects over the age of 80 suggest that while the doses of benzodiazepine used to induce sedation should be reduced, ordinary doses of flumazenil may be used for reversal.
Gender
The pharmacokinetics of flumazenil are not different in male and female subjects.
Race
There is no FDA guidance on the use of Flumazenil with respect to specific racial populations.
Renal Impairment
In renal Failure (creatinine clearance <10 mL/min) and Hemodialysis the pharmacokinetics of flumazenil are not significantly affected.
Hepatic Impairment
For patients with moderate liver dysfunction, their mean total clearance is decreased to 40% to 60% and in patients with severe liver dysfunction, it is decreased to 25% of normal value, compared with age-matched healthy subjects. This results in a prolongation of the half-life to 1.3 hours in patients with moderate hepatic impairment and 2.4 hours in severely impaired patients. Caution should be exercised with initial and/or repeated dosing to patients with liver disease.
Females of Reproductive Potential and Males
A reproduction study in male and female rats did not show any impairment of fertility at oral dosages of 125 mg/kg/day. From the available data on the area under the curve (AUC) in animals and man the dose represented 120 times the human exposure from a maximum recommended intravenous dose of 5 mg.
Immunocompromised Patients
There is no FDA guidance one the use of Flumazenil in patients who are immunocompromised.
Psychiatric Patients
Flumazenil has been reported to provoke panic attacks in patients with a history of panic disorder.
Administration and Monitoring
Administration
Intravenous
Monitoring
There is limited information regarding Flumazenil Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Flumazenil and IV administrations.
Overdosage
There is limited experience of acute overdose with flumazenil.
There is no specific antidote for overdose with flumazenil. Treatment of an overdose with flumazenil should consist of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient.
Intravenous bolus administration of doses ranging from 2.5 to 100 mg (exceeding those recommended) of flumazenil, when administered to healthy normal volunteers in the absence of a benzodiazepine agonist, produced no serious adverse reactions, severe signs or symptoms, or clinically significant laboratory test abnormalities. In clinical studies, most adverse reactions to flumazenil were an extension of the pharmacologic effects of the drug in reversing benzodiazepine effects.
Reversal with an excessively high dose of flumazenil may produce anxiety, agitation, increased muscle tone, hyperesthesia and possibly convulsions. Convulsions have been treated with barbiturates, benzodiazepines and phenytoin, generally with prompt resolution of the seizures
Pharmacology
Mechanism of Action
Flumazenil, an imidazobenzodiazepine derivative, antagonizes the actions of benzodiazepines on the central nervous system. Flumazenil competitively inhibits the activity at the benzodiazepine recognition site on the GABA/benzodiazepine receptor complex. Flumazenil is a weak partial agonist in some animal models of activity, but has little or no agonist activity in man.
Flumazenil does not antagonize the central nervous system effects of drugs affecting GABA-ergic neurons by means other than the benzodiazepine receptor (including ethanol, barbiturates, or general anesthetics) and does not reverse the effects of opioids.
In animals pretreated with high doses of benzodiazepines over several weeks, flumazenil elicited symptoms of benzodiazepine withdrawal, including seizures. A similar effect was seen in adult human subjects.
Structure
Flumazenil is ethyl 8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a](1,4) benzodiazepine-3-carboxylate. Flumazenil has an imidazobenzodiazepine structure, a calculated molecular weight of 303.3 and the following structural formula:
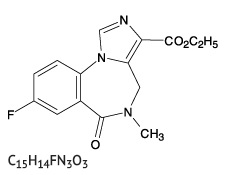
Flumazenil is a white to off-white crystalline compound with an octanol:buffer partition coefficient of 14 to 1 at pH 7.4. It is insoluble in water but slightly soluble in acidic aqueous solutions.
Pharmacodynamics
Intravenous flumazenil has been shown to antagonize sedation, impairment of recall, psychomotor impairment and ventilatory depression produced by benzodiazepines in healthy human volunteers.
The duration and degree of reversal of sedative benzodiazepine effects are related to the dose and plasma concentrations of flumazenil as shown in the following data from a study in normal volunteers.

Generally, doses of approximately 0.1 mg to 0.2 mg (corresponding to peak plasma levels of 3 to 6 ng/mL) produce partial antagonism, whereas higher doses of 0.4 to 1 mg (peak plasma levels of 12 to 28 ng/mL) usually produce complete antagonism in patients who have received the usual sedating doses of benzodiazepines. The onset of reversal is usually evident within 1 to 2 minutes after the injection is completed. Eighty percent response will be reached within 3 minutes, with the peak effect occurring at 6 to 10 minutes. The duration and degree of reversal are related to the plasma concentration of the sedating benzodiazepine as well as the dose of flumazenil given.
In healthy volunteers, flumazenil did not alter intraocular pressure when given alone and reversed the decrease in intraocular pressure seen after administration of midazolam.
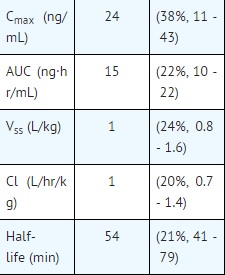
Pharmacokinetics
After IV administration, plasma concentrations of flumazenil follow a two-exponential decay model. The pharmacokinetics of flumazenil are dose-proportional up to 100 mg.
Distribution
Flumazenil is extensively distributed in the extravascular space with an initial distribution half-life of 4 to 11 minutes and a terminal half-life of 40 to 80 minutes. Peak concentrations of flumazenil are proportional to dose, with an apparent initial volume of distribution of 0.5 L/kg. The volume of distribution at steady-state is 0.9 to 1.1 L/kg. Flumazenil is a weak lipophilic base. Protein binding is approximately 50% and the drug shows no preferential partitioning into red blood cells. Albumin accounts for two thirds of plasma protein binding.
Metabolism
Flumazenil is completely (99%) metabolized. Very little unchanged flumazenil (<1%) is found in the urine. The major metabolites of flumazenil identified in urine are the de-ethylated free acid and its glucuronide conjugate. In preclinical studies there was no evidence of pharmacologic activity exhibited by the de-ethylated free acid.
Elimination
Elimination of radiolabeled drug is essentially complete within 72 hours, with 90% to 95% of the radioactivity appearing in urine and 5% to 10% in the feces. Clearance of flumazenil occurs primarily by hepatic metabolism and is dependent on hepatic blood flow. In pharmacokinetic studies of normal volunteers, total clearance ranged from 0.8 to 1.0 L/hr/kg.
Pharmacokinetic parameters following a 5 minute infusion of a total of 1 mg of flumazenil mean (coefficient of variation, range):
Nonclinical Toxicology
There is limited information regarding Flumazenil Nonclinical Toxicology in the drug label.
Clinical Studies
Flumazenil has been administered in adults to reverse the effects of benzodiazepines in conscious sedation, general anesthesia, and the management of suspected benzodiazepine overdose. Limited information from uncontrolled studies in pediatric patients is available regarding the use of flumazenil to reverse the effects of benzodiazepines in conscious sedation only.
Conscious Sedation in Adults
- Flumazenil was studied in four trials in 970 patients who received an average of 30 mg diazepam or 10 mg midazolam for sedation (with or without a narcotic) in conjunction with both inpatient and outpatient diagnostic or surgical procedures. Flumazenil was effective in reversing the sedating and psychomotor effects of the benzodiazepine; however, amnesia was less completely and less consistently reversed. In these studies, flumazenil was administered as an initial dose of 0.4 mg IV (two doses of 0.2 mg) with additional 0.2 mg doses as needed to achieve complete awakening, up to a maximum total dose of 1 mg.
- Seventy-eight percent of patients receiving flumazenil responded by becoming completely alert. Of those patients, approximately half responded to doses of 0.4 mg to 0.6 mg, while the other half responded to doses of 0.8 mg to 1 mg. Adverse effects were infrequent in patients who received 1 mg of flumazenil or less, although injection site pain, agitation, and anxiety did occur. Reversal of sedation was not associated with any increase in the frequency of inadequate analgesia or increase in narcotic demand in these studies. While most patients remained alert throughout the 3 hour postprocedure observation period, resedation was observed to occur in 3% to 9% of the patients, and was most common in patients who had received high doses of benzodiazepines.
General Anesthesia in Adults
- Flumazenil was studied in four trials in 644 patients who received midazolam as an induction and/or maintenance agent in both balanced and inhalational anesthesia. Midazolam was generally administered in doses ranging from 5 mg to 80 mg, alone and/or in conjunction with muscle relaxants, nitrous oxide, regional or local anesthetics, narcotics and/or inhalational anesthetics. Flumazenil was given as an initial dose of 0.2 mg IV, with additional 0.2 mg doses as needed to reach a complete response, up to a maximum total dose of 1 mg. These doses were effective in reversing sedation and restoring psychomotor function, but did not completely restore memory as tested by picture recall. Flumazenil was not as effective in the reversal of sedation in patients who had received multiple anesthetic agents in addition to benzodiazepines.
- Eighty-one percent of patients sedated with midazolam responded to flumazenil by becoming completely alert or just slightly drowsy. Of those patients, 36% responded to doses of 0.4 mg to 0.6 mg, while 64% responded to doses of 0.8 mg to 1 mg.
- Resedation in patients who responded to flumazenil occurred in 10% to 15% of patients studied and was more common with larger doses of midazolam (>20 mg), long procedures (>60 minutes) and use of neuromuscular blocking agents (see PRECAUTIONS).
Management of Suspected Benzodiazepine Overdose in Adults
- Flumazenil was studied in two trials in 497 patients who were presumed to have taken an overdose of a benzodiazepine, either alone or in combination with a variety of other agents. In these trials, 299 patients were proven to have taken a benzodiazepine as part of the overdose, and 80% of the 148 who received flumazenil responded by an improvement in level of consciousness. Of the patients who responded to flumazenil, 75% responded to a total dose of 1 mg to 3 mg.
- Reversal of sedation was associated with an increased frequency of symptoms of CNS excitation. Of the patients treated with flumazenil, 1% to 3% were treated for agitation or anxiety. Serious side effects were uncommon, but six seizures were observed in 446 patients treated with flumazenil in these studies. Four of these 6 patients had ingested a large dose of cyclic antidepressants, which increased the risk of seizures
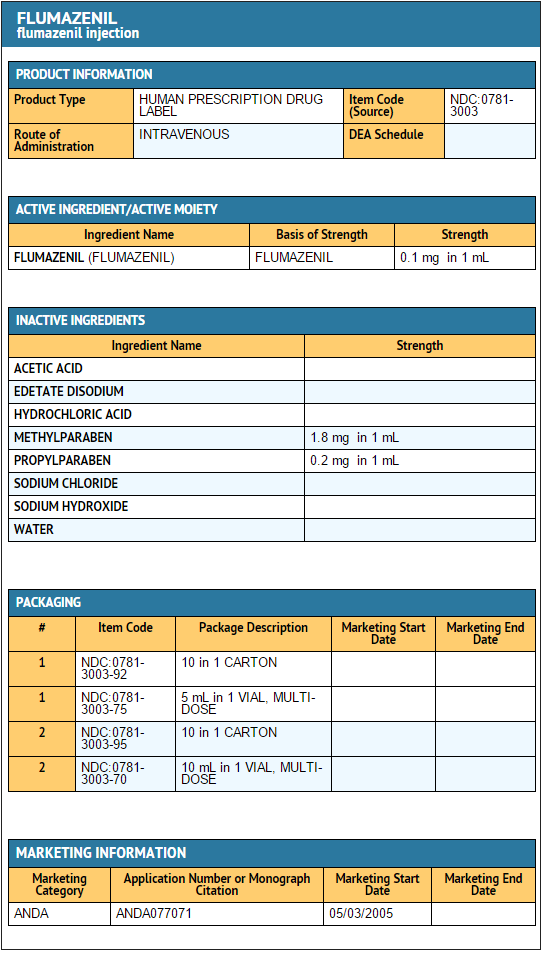
How Supplied
Flumazenil injection contains 0.1 mg of flumazenil per mL and is supplied as follows:
- NDC 0781-3003-92 multiple-dose vials of 5 mL in boxes of 10.
- NDC 0781-3003-95 multiple-dose vials of 10 mL in boxes of 10.
Storage
Store at 20° to 25°C (68° to 77°F)
Images
Drug Images
{{#ask: Page Name::Flumazenil |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Flumazenil |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Flumazenil does not consistently reverse amnesia. Patients cannot be expected to remember information told to them in the postprocedure period and instructions given to patients should be reinforced in writing or given to a responsible family member. Physicians are advised to discuss with patients or their guardians, both before surgery and at discharge, that although the patient may feel alert at the time of discharge, the effects of the benzodiazepine (e.g., sedation) may recur. As a result, the patient should be instructed, preferably in writing, that their memory and judgment may be impaired and specifically advised:
- Not to engage in any activities requiring complete alertness, and not to operate hazardous machinery or a motor vehicle during the first 24 hours after discharge, and it is certain no residual sedative effects of the benzodiazepine remain.
- Not to take any alcohol or non-prescription drugs during the first 24 hours after flumazenil administration or if the effects of the benzodiazepine persist.
Precautions with Alcohol
Flumazenil should be used with caution in patients with alcoholism and other drug dependencies due to the increased frequency of benzodiazepine tolerance and dependence observed in these patient populations.
Flumazenil is not recommended either as a treatment for benzodiazepine dependence or for the management of protracted benzodiazepine abstinence syndromes, as such use has not been studied.
The administration of flumazenil can precipitate benzodiazepine withdrawal in animals and man. This has been seen in healthy volunteers treated with therapeutic doses of oral lorazepam for up to 2 weeks who exhibited effects such as hot flushes, agitation and tremor when treated with cumulative doses of up to 3 mg doses of flumazenil.
Similar adverse experiences suggestive of flumazenil precipitation of benzodiazepine withdrawal have occurred in some adult patients in clinical trials. Such patients had a short-lived syndrome characterized by dizziness, mild confusion, emotional lability, agitation (with signs and symptoms of anxiety), and mild sensory distortions. This response was dose-related, most common at doses above 1 mg, rarely required treatment other than reassurance and was usually short lived. When required, these patients (5 to 10 cases) were successfully treated with usual doses of a barbiturate, a benzodiazepine, or other sedative drug.
Practitioners should assume that flumazenil administration may trigger dose-dependent withdrawal syndromes in patients with established physical dependence on benzodiazepines and may complicate the management of withdrawal syndromes for alcohol, barbiturates and cross- tolerant sedatives.
Brand Names
- Romazicon [1]
Look-Alike Drug Names
There is limited information regarding Flumazenil Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Flumazenil |Label Name=Flumazenil 0.5 mg.jpg
}}