Bemegride
 | |
| Clinical data | |
|---|---|
| Trade names | Mikedimide (Panray), Eukraton (Nordmark), Malysol (Arco, Switzerland), Megimide (Nicholas) |
| Synonyms | Methetharimide β,β-methylethylglutarimide |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
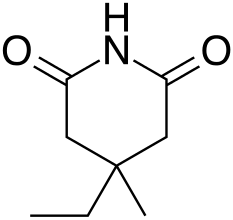
| Formula | C8H13NO2 |
| Molar mass | 155.194 g/mol |
| 3D model (JSmol) | |
| Melting point | 127 °C (260.6 °F) |
| |
| |
| (verify) | |
|
WikiDoc Resources for Bemegride |
|
Articles |
|---|
|
Most recent articles on Bemegride |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Bemegride at Clinical Trials.gov Clinical Trials on Bemegride at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Bemegride
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Bemegride Discussion groups on Bemegride Directions to Hospitals Treating Bemegride Risk calculators and risk factors for Bemegride
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Bemegride |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Bemegride (also marketed as Megimide) is a central nervous system stimulant and antidote for barbiturate poisoning[1] as its chemoreceptor agonism increases mean tidal volume, thereby increasing respiration and the concentration of O2 in blood although it may be theoretically used as a supportive measure in treating any depressant overdose. The drug's synthesis was invented in 1911.[2]
John Bodkin Adams case
Bemegride is notable in legal history as the drug suspected serial killer Dr John Bodkin Adams failed to prescribe correctly to his patient Gertrude Hullett. Hullett took an overdose of barbiturates on 19 July 1956 but Adams only gave her a single 10cc dose of bemegride three days later on the 22nd, despite having acquired 100cc for her treatment. Hullett died the next day on 23 July 1956. Adams was charged but never tried for her murder.[3]
Animal use
Bemegride is also used to induce convulsions in experimental animals.[4]
Synthesis

The original synthesis involves first the condensation of methylethylketone with two equivalents of cyanoacetamide. The product can be rationalized by assuming first aldol condensation of ketone and active methylene compound followed by dehydration to give 3. Conjugate addition of a second molecule of cyanoacetamide would afford 4. Addition of one of the amide amines to the nitrile would then afford the iminonitrile 5. The observed product 6 can be rationalized by assuming loss of the carboxamide under strongly basic conditions. Decarboxylative hydrolysis of 6 then leads to bemigride 7.
References
- ↑ Hofmeister, Alfred (2000). "Analeptics". Ullmann's Encyclopedia of Industrial Chemistry: 1–2. doi:10.1002/14356007.a02_267.
- ↑ 2.0 2.1 Thole, Ferdinand Bernard; Thorpe, Jocelyn Field (1911). "LIII.—The formation and reactions of iminocompounds. Part XV. The production of imino-derivatives of piperidine leading to the formation of the ββ-disubstituted glutaric acids". Journal of the Chemical Society, Transactions. 99: 422–448. doi:10.1039/CT9119900422.
- ↑ Cullen, Pamela V., A Stranger in Blood: The Case Files on Dr John Bodkin Adams, London, Elliott & Thompson, 2006, ISBN 1-904027-19-9
- ↑ Definition: bemegride from Online Medical Dictionary
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Drugboxes which contain changes to watched fields
- Antidotes
- Stimulants
- Respiratory agents
- Drug