Calcium gluconate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Calcium gluconate is a mineral supplement that is FDA approved for the treatment of hypocalcemic tetany, hypocalcemia, black widow spider bites to relieve muscle cramping, rickets, osteomalacia, lead colic and magnesium sulfate overdosage, non thrombocytopnic pupura, exudative dermatoses, pruritus, hyperkalemia. Common adverse reactions include tingling sensations, a sense of oppression or heat waves and a calcium or chalky taste, vasodilation, hypotension, bradycardia, cardiac arrhythmias, syncope and cardiac arrest.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Calcium Gluconate Injection, USP is used to treat conditions arising from calcium deficiencies such as hypocalcemic tetany, hypocalcemia related to hypoparathyroidism and hypocalcemia due to rapid growth of pregnancy.
- It is also used in the treatment of black widow spider bites to relieve muscle cramping and as an adjunct in the treatment of rickets, osteomalacia, lead colic and magnesium sulfate overdosage.
- Calcium gluconate has also been employed to decrease capillary permeability in allergic conditions, non thrombocytopnic pupura and exudative dermatoses such as dermatitis herpetiformis and for pruritus of eruptions caused by certain drugs. In hyperkalemia, calcium gluconate may aid in antagonizing the cardiac toxicity, provided the patient is not receiving digitalis therapy.
Dosage
- The dose is dependent on the requirements of the individual patient. Intravenous calcium gluconate injection must be administered slowly.
- Adults-500 mg to 2 g (5 to 20 mL)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Calcium Gluconate in adult patients.
Non–Guideline-Supported Use
Indications
Allergic condition
Cardiac arrest, due to hyperkalemia
Cardiac arrest, due to hypermagnesemia
Chemical burn, Hydrofluoric acid
Dermatitis, exudative
Tetany, due to hypocalcemia
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications
- Calcium Gluconate Injection, USP is used to treat conditions arising from calcium deficiencies such as hypocalcemic tetany, hypocalcemia related to hypoparathyroidism and hypocalcemia due to rapid growth of pregnancy.
- It is also used in the treatment of black widow spider bites to relieve muscle cramping and as an adjunct in the treatment of rickets, osteomalacia, lead colic and magnesium sulfate overdosage.
- Calcium gluconate has also been employed to decrease capillary permeability in allergic conditions, non thrombocytopnic pupura and exudative dermatoses such as dermatitis herpetiformis and for pruritus of eruptions caused by certain drugs. In hyperkalemia, calcium gluconate may aid in antagonizing the cardiac toxicity, provided the patient is not receiving digitalis therapy.
Dosage
- Pediatric Patients-200 to 500 mg (2 to 5 mL)
- Infants-Not more than 200 mg (not more than 2 mL)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Calcium Gluconate in pediatric patients.
Non–Guideline-Supported Use
Indications
- Rickets
- Allergic condition
- Cardiac arrest, due to hyperkalemia
- Cardiac arrest, due to hypermagnesemia
- Dermatitis, exudative
Contraindications
Calcium salts are contraindicated in patients with ventricular fibrillation or hypercalcemia. Intravenous administration of calcium is contraindicated when serum calcium levels are above normal.
Warnings
- For intravenous use only. Subcutaneous or intramuscular injection may cause severe necrosis and sloughing.
- This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
- Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
Adverse Reactions
Clinical Trials Experience
- Patients may complain of tingling sensations, a sense of oppression or heat waves and a calcium or chalky taste following the intravenous administration of calcium gluconate.
- Rapid intravenous injection of calcium salts may cause vasodilation, decreased blood pressure, bradycardia, cardiac arrhythmias, syncope and cardiac arrest. Use in digitalized patients may precipitate arrhythmias.
- Local necrosis and abscess formation may occur with intramuscular injection.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Calcium Gluconate in the drug label.
Drug Interactions
- The inotropic and toxic effects of cardiac glycosides and calcium are synergistic, and arrhythmias may occur if these drugs are given together (particularly when calcium is given together particularly when calcium is given intravenously). Intravenous administration of calcium should be avoided in patients receiving cardiac glycosides; if necessary, calcium should be given slowly in small amounts.
- Calcium complexes tetracycline antibiotics rendering them inactive. The two drugs should not be given at the same time orally nor should they be mixed for parenteral administration.
- Calcium gluconate injection has been reported to be incompatible with intravenous solutions containing various drugs. Published data are too varied and/or limited to permit generalizations, and specialized reference should be consulted for specific information.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
Teratogenic Effects: Pregnancy Category C- Animal reproduction studies have not been conducted with calcium gluconate. It is also not known whether calcium gluconate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Calcium gluconate should be given to a pregnant woman only if clearly needed.
Pregnancy Category (AUS):
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Calcium gluconate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Calcium gluconate during labor and delivery.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when calcium gluconate is administered to a nursing woman.
Pediatric Use
There is no FDA guidance on the use of Calcium gluconate with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Calcium gluconate with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Calcium gluconate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Calcium gluconate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Calcium gluconate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Calcium gluconate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Calcium gluconate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Calcium gluconate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
There is limited information regarding Monitoring of Calcium gluconate in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Calcium Gluconate in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Calcium Gluconate in the drug label.
Pharmacology
There is limited information regarding Calcium gluconate Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Calcium gluconate Mechanism of Action in the drug label.
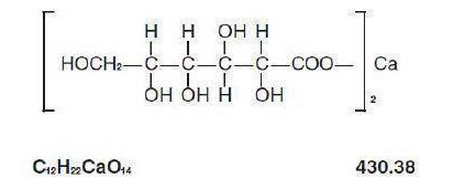
Structure
Calcium Gluconate Injection, USP is a sterile, nonpyrogenic, supersaturated solution of calcium gluconate for intravenous use only.
Each mL contains: Calcium gluconate 94 mg; calcium saccharate (tetrahydrate) 4.5 mg; Water for Injection q.s. Hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment (6.0-8.2)
Calcium saccharate provides 6% of the total calcium and stabilizes the supersaturated solution of calcium gluconate.
Each 10 mL of the injection provides 93 mg elemental calcium (Ca++) equivalent to 1 g of calcium gluconate.
The structural formula is:
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Calcium Gluconate in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Calcium Gluconate in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Calcium Gluconate in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Calcium Gluconate in the drug label.
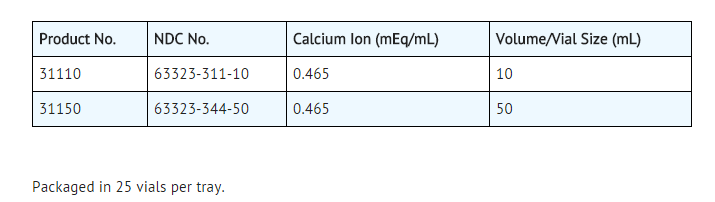
How Supplied
Storage
- Store at 20o to 25o C (68o to 77o F). Do not permit to freeze.
Images
Drug Images
{{#ask: Page Name::Calcium gluconate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Calcium gluconate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Calcium Gluconate in the drug label.
Precautions with Alcohol
- Alcohol-Calcium Gluconate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Calcium gluconate Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Calcium gluconate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Calcium gluconate
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Calcium gluconate |Label Name=Calcium gluconate11.png
}}
{{#subobject:
|Label Page=Calcium gluconate |Label Name=Calcium gluconate11.png
}}