|
|
| (22 intermediate revisions by 8 users not shown) |
| Line 1: |
Line 1: |
| | __NOTOC__ |
| {{Infobox_Disease | | | {{Infobox_Disease | |
| Name = Coeliac disease | | | Name = Celiac disease | |
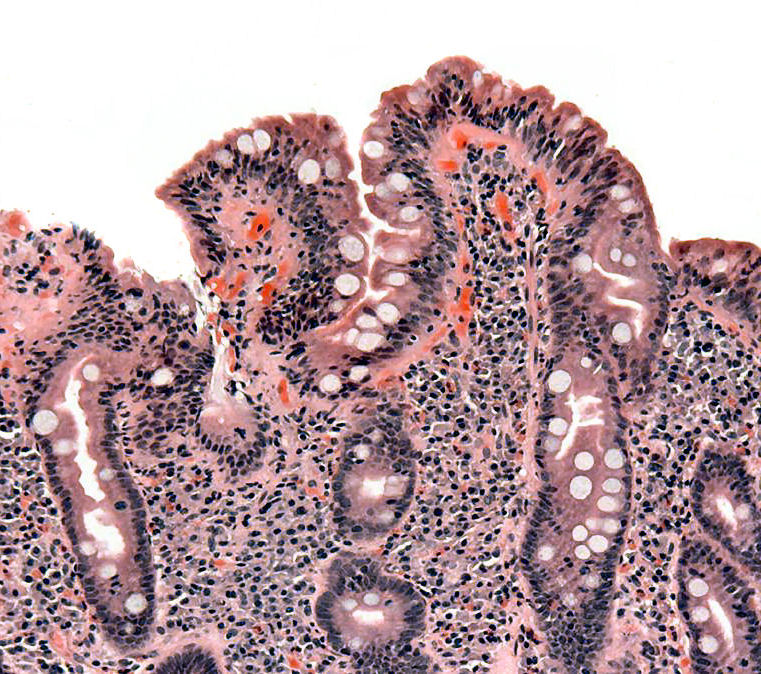
| Image = Coeliac path.jpg| | | Image = Coeliac path.jpg| |
| Caption = Biopsy of [[small bowel]] showing coeliac disease manifested by blunting of [[intestinal villus|villi]], crypt hyperplasia, and [[lymphocyte]] infiltration of crypts. | | | Caption = Biopsy of [[small bowel]] showing celiac disease manifested by blunting of [[intestinal villus|villi]], crypt hyperplasia, and [[lymphocyte]] infiltration of crypts. | |
| DiseasesDB = 2922 |
| |
| ICD10 = {{ICD10|K|90|0|k|90}} |
| |
| ICD9 = {{ICD9|579.0}} |
| |
| ICDO = |
| |
| OMIM = 212750 |
| |
| MedlinePlus = 000233 |
| |
| eMedicineSubj = |
| |
| eMedicineTopic = |
| |
| eMedicine_mult = |
| |
| MeshID = D002446 |
| |
| }} | | }} |
| | |
| | {{Celiac disease}} |
| '''For patient information click [[{{PAGENAME}} (patient information)|here]]''' | | '''For patient information click [[{{PAGENAME}} (patient information)|here]]''' |
| {{SI}}
| |
| {{CMG}}
| |
|
| |
| ==Overview==
| |
| '''Coeliac disease''', also spelled '''celiac disease''', is an [[Autoimmunity|autoimmune]] disorder of the [[small bowel]] that occurs in [[Genetic predisposition|genetically predisposed]] people of all ages from middle infancy. Symptoms include chronic [[diarrhoea]], [[failure to thrive]] (in children) and [[fatigue (physical)|fatigue]], but these may be absent and symptoms in all other organ systems have been described. It is estimated to affect about 1% of Indo-European populations, although significantly underdiagnosed. A growing portion of diagnoses are being made in asymptomatic persons as a result of increasing screening.<ref name=VanHeelWest>{{cite journal | author = van Heel D, West J | title = Recent advances in coeliac disease | url = http://gut.bmjjournals.com/cgi/content/full/55/7/1037 | journal = Gut | volume = 55 | issue = 7 | pages = 1037–46 | year = 2006 | id = PMID 16766754}}</ref>
| |
|
| |
| ==Background==
| |
| Coeliac disease is caused by a reaction to [[gliadin]], a [[gluten]] protein found in [[wheat]] (and similar proteins of the tribe Triticeae which includes other cultivars such as barley and rye). Upon exposure to gliadin, the enzyme [[tissue transglutaminase]] modifies the protein, and the [[immune system]] cross-reacts with the bowel tissue, causing an [[inflammation|inflammatory reaction]]. That leads to flattening of the lining of the small intestine, which [[malabsorption|interferes with the absorption]] of nutrients. The only effective treatment is a lifelong [[gluten-free diet]].
| |
|
| |
| This condition has several other names, including: '''cœliac disease''' (with "œ" ligature), '''c(o)eliac sprue''', '''non-tropical sprue''', '''endemic sprue''', '''gluten enteropathy''' or '''gluten-sensitive enteropathy''', and '''gluten intolerance'''. The term ''coeliac'' derives from the Greek κοιλιακος (''koiliakos'', abdominal), and was introduced in the 19th century in a translation of what is generally regarded as an ancient Greek description of the disease by [[Aretaeus of Cappadocia]].<ref name="Aretaeus">{{cite book |last=Adams F, translator |title=The extant works of Aretaeus, The Cappadocian |url=http://web.archive.org/web/20070311164628/http://www.chlt.org/sandbox/dh/aretaeusEnglish/index.html |chapter= On The Cœliac Affection |chapterurl=http://web.archive.org/web/20070311164628/http://www.chlt.org/sandbox/dh/aretaeusEnglish/page.102.a.php |accessdate=2006-09-04 |year=1856 |publisher=Sydenham Society |location=London}} See also [http://books.google.com/books?id=v4gIAAAAIAAJ&pg=PP1&ct=title#PRA1-PA350,M1 Google Books entry]</ref>
| |
|
| |
| ==Definition==
| |
| American Gastroenterological Association (AGA) Definition: “Chronic malabsorptive disorder of the small intestine caused by exposure to dietary gluten in genetically predisposed individuals”
| |
|
| |
| ==History==
| |
| [[Aretaeus of Cappadocia]], living in the second century, recorded a malabsorptive syndrome with chronic diarrhoea. His "Cœliac Affection" is a translation of the [[Greek language|Greek]] κοιλιακος (''koiliakos'', abdominal). It gained the attention of Western medicine when [[Francis Adams (translator)|Francis Adams]] presented a translation of Aretaeus' work at the Sydenham Society in 1856. The problem, Aretaeus believed, was a lack of heat in the stomach necessary to digest the food and a reduced ability to distribute the digestive products throughout the body. This incomplete digestion resulted in loose stools that were white, malodorous and flatulent. The patient had stomach pain and was atrophied, pale, feeble and incapable of work. The disease was intractable and liable to periodic return. He regarded this as an affliction of the old and more commonly affecting women, explicitly excluding children. The cause, according to Aretaeus, was sometimes either another chronic disease or even consuming "a copious draught of cold water".<ref name="Aretaeus"/>
| |
|
| |
|
| The [[pediatrics|paediatrician]] [[Samuel Gee]] gave the first modern-day description of the condition in a lecture at [[Great Ormond Street Hospital|Hospital for Sick Children, Great Ormond Street]], London in 1887. Gee acknowledges earlier descriptions and terms for the disease and adopts the same term as Aretaeus. Unlike Aretaeus, he includes children in the scope of the affection, particularly those between one and five years old. Gee finds the cause to be obscure and fails to spot anything abnormal during post-mortem examination (the lining of the small bowel quickly deteriorates on death).<ref name=Holmes2006>{{cite web | url = http://www.coeliac.co.uk/coeliac_disease/68.asp | title = History of coeliac disease | accessdate = 2007-03-20 | last = Holmes | first = Geoff | year = 2006 | publisher = Coeliac UK}}</ref> He perceptively states "if the patient can be cured at all, it must be by means of diet." Gee recognises that milk intolerance is a problem with coeliac children and that highly starched foods should be avoided. He forbids rice, sago, fruit and vegetables, which all would have been safe to eat. Raw meat is recommended as are thin slices of toasted bread. Gee highlights particular success with a child "who was fed upon a quart of the best Dutch mussels daily". However, the child cannot bear this diet for more than one season.<ref>{{cite journal | last = Gee | first = SJ | year = 1888 | title = On the coeliac affection | journal = St Bartholomew's Hospital Report | volume = 24 | pages = 17–20 | url = http://web2.bium.univ-paris5.fr/livanc/?cote=epo0466&p=1&do=page}}</ref>
| | {{CMG}}; {{AE}} {{MMF}}, {{MIR}}, {{SMP}}, {{USAMA}}, {{HK}}, {{ADG}}, {{Akshun}}, {{IQ}}, {{Anmol}}, {{EG}}, {{AY}}, {{Ajay}}, {{AEL}}, {{RHN}} |
|
| |
|
| [[Christian Archibald Herter (physician)|Christian Archibald Herter]], an American physician, wrote a book in 1908 on children with coeliac disease, which he called "intestinal infantilism". He noted their growth was retarded and that fat was better tolerated than carbohydrate. The [[eponym]] Gee-Herter disease was sometimes used to acknowledge both contributions.<ref name=Herter1908>{{cite book | last = Herter | first = CA | year = 1908 | title = On infantilism from chronic intestinal infection; characterized by the overgrowth and persistence of flora in the nursing period | publisher = Macmillan & Co | location = New York}} as cited by WhoNamedIt</ref><ref name=whoNamedItHerter> {{cite web | url = http://www.whonamedit.com/doctor.cfm/1490.html | title = Christian Archibald Herter | accessdate = 2007-03-20 | author = Ole Daniel Enersen | publisher = Who Named It?}}</ref> Sydney V. Haas, an American paediatrician, reported positive effects of a diet of bananas in 1924.<ref>Haas SV (1924). The value of the banana in the treatment of coeliac disease. ''Am J Dis Child'' '''24''': 421–37.</ref> This diet remained in vogue until the actual cause of coeliac disease was determined.
| | {{SK}} Coeliac disease; Celiac sprue; Gee-Herter-Heubner disease; Gluten sensitive enteropathy; GSE; Nontropical sprue. |
|
| |
|
| While a role for carbohydrates had been suspected, the link with wheat was not made until 1950 by the Dutch paediatrician Dr Willem Dicke.<ref>{{cite journal | author = van Berge-Henegouwen G, Mulder C | title = Pioneer in the gluten free diet: Willem-Karel Dicke 1905–1962, over 50 years of gluten free diet | journal = Gut | volume = 34 | issue = 11 | pages = 1473–5 | year = 1993 | id = PMID 8244125}}</ref> It is likely that clinical improvement of his patients during the Dutch famine of 1944 (during which flour was sparse) may have contributed to his discovery.<ref>Dicke WK. ''Coeliakie: een onderzoek naar de nadelige invloed van sommige graansoorten op de lijder aan coeliakie'' [PhD thesis]. Utrecht, the Netherlands: University of Utrecht, 1950.</ref> The link with the gluten component of wheat was made in 1952 by a team from Birmingham, England.<ref>{{cite journal | author = Anderson C, French J, Sammons H, Frazer A, Gerrard J, Smellie J | title = Coeliac disease; gastrointestinal studies and the effect of dietary wheat flour | journal = Lancet | volume = 1 | issue = 17 | pages = 836-42 | year = 1952 | id = PMID 14918439}}</ref> Villous atrophy was described by British physician John W. Paulley in 1954.<ref>{{cite journal | author = Paulley JW | title = Observation on the aetiology of idiopathic steatorrhoea; jejunal and lymph-node biopsies | journal = Br Med J | volume = 4900 | issue = | pages = 1318–21 | year =|id = PMID 13209109}}</ref> Paulley was able to examine biopsies taken from patients during abdominal operations.<ref name=Holmes2006/> Dr Margo Shiner, working on Prof Sheila Sherlock's team at the Postgraduate Medical School in London, described the principles of small bowel biopsy in 1956.<ref>{{cite journal | author = Shiner M | title = Duodenal biopsy | journal = Lancet | volume = 270 | issue = 6906 | pages = 17-9 | year = 1956 | id = PMID 13279152}}</ref>
| | ==[[Celiac disease overview|Overview]]== |
|
| |
|
| Throughout the 1960s other features of coeliac disease were elucidated. Its hereditary character was recognized in 1965.<ref>{{cite journal |author=Macdonald W, Dobbins W, Rubin C |title=Studies of the familial nature of celiac sprue using biopsy of the small intestine |journal=N Engl J Med |volume=272 |issue= |pages=448-56 |year=1965 |pmid=14242522}}</ref> In 1966 [[dermatitis herpetiformis]] was linked to gluten sensitivity.<ref name=Marks/> The link with tissue [[transglutaminase]] was not made until 1997.<ref name=Dieterich/>
| | ==[[Celiac disease historical perspective|Historical Perspective]]== |
|
| |
|
| == Natural History == | | ==[[Celiac disease classification|Classification]]== |
| === Sequelae include ===
| |
| * [[B12 deficiency]]
| |
| * [[Iron deficiency]]
| |
| * [[Folate deficiency]]
| |
| * [[Anemia]]
| |
| * [[Hypoalbuminemia]]
| |
| * [[Osteoporosis]]
| |
| * [[Osteomalacia]]
| |
| * Increased risk of small bowel [[lymphoma]] (falls to nl after 5y gluten-free diet)
| |
|
| |
|
| ==Prevalence== | | ==[[Celiac disease pathophysiology|Pathophysiology]]== |
| Celiac Disease is more prevalent than previously thought. Prevalence has been shown to be as high as 1:250 <ref>Detecting Celiac Disease in Your Patients", American Family Physician, Vol. 57/No. 5, Pruessner, Harold T., M.D. http://www.aafp.org/afp/980301ap/pruessn.html</ref> ). The prevalence may be as high as 1:133 in the general population.
| |
|
| |
|
| ==Epidemiology== | | ==[[Celiac disease causes|Causes]]== |
| The prevalence of clinically diagnosed disease (symptoms prompting diagnostic testing) is 0.05–0.27% in various studies. However, population studies from parts of Europe, India, South America, Australasia and the USA (using serology and biopsy) indicate that the prevalence may be between 0.33 and 1.06% in children (5.66% in one study of Saharawi children<ref name="Catassi1999">{{cite journal | author = Catassi C, Rätsch I, Gandolfi L, Pratesi R, Fabiani E, El Asmar R, Frijia M, Bearzi I, Vizzoni L | title = Why is coeliac disease endemic in the people of the Sahara? | journal = Lancet | volume = 354 | issue = 9179 | pages = 647–8 | year = 1999|id = PMID 10466670}}</ref>) and 0.18–1.2% in adults.<ref name=VanHeelWest/> People of African, Japanese and Chinese descent are rarely diagnosed; this reflects a much lower prevalence of the genetic risk factors. Population studies also indicate that a large proportion of coeliacs remain undiagnosed; this is due to many clinicians being unfamiliar with the condition.<ref>{{cite journal |author=Zipser R, Farid M, Baisch D, Patel B, Patel D |title=Physician awareness of celiac disease: a need for further education |journal=J Gen Intern Med |volume=20 |issue=7 |pages=644-6 |year=2005 |pmid=16050861}}</ref>
| |
|
| |
|
| A large multicentre study in the U.S. found a prevalence of 0.75% in not-at-risk groups, rising to 1.8% in symptomatic patients, 2.6% in second-degree relatives of a patient with coeliac disease and 4.5% in first-degree relatives. This profile is similar to the prevalence in Europe.<ref name="Fasano2003">{{cite journal | author = Fasano A, Berti I, Gerarduzzi T, Not T, Colletti R, Drago S, Elitsur Y, Green P, Guandalini S, Hill I, Pietzak M, Ventura A, Thorpe M, Kryszak D, Fornaroli F, Wasserman S, Murray J, Horvath K | title = Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study | journal = Archives of Internal Medicine | volume = 163 | issue = 3 | pages = 286–92 | year = 2003 | url = http://archinte.ama-assn.org/cgi/content/full/163/3/286 | id = PMID 12578508}}</ref>
| | ==[[Differentiating celiac disease from other diseases|Differentiating Celiac Disease from other Diseases]]== |
|
| |
|
| ==Pathophysiology== | | ==[[Celiac disease epidemiology and demographics|Epidemiology and Demographics]]== |
| Coeliac disease appears to be polyfactorial, both in that more than one abnormal factor can cause the disease and also more than one factor is necessary for the disease to manifest in a patient.
| |
|
| |
|
| Most all coeliac patients have abnormal [[HLA DQ]]2 [[allele]].<ref name=VanHeelWest/> However, about 20–30% of people without coeliac disease have inherited an abnormal [[HLA-DQ]]2 [[allele]].<ref name="pmid17785484"/> This suggests additional factors are needed for coeliac disease to develop. Furthermore, about 5% of those people who do develop coeliac disease do not have the DQ2 gene.<ref name=VanHeelWest/>
| | ==[[Celiac disease risk factors|Risk Factors]]== |
|
| |
|
| The [[HLA-DQ]]2 [[allele]] shows incomplete [[penetrance]], as the gene [[allele]]s associated with the disease appear in most patients, but are neither present in all cases nor sufficient by themselves cause the disease.
| | ==[[Celiac disease screening|Screening]]== |
|
| |
|
| === Associated Conditions === | | ==[[Celiac disease natural history|Natural History, Complications and Prognosis]]== |
| * Insulin dependent diabetes mellitus (IDDM)
| |
| * Sjogren’s syndrome
| |
| * Thyroid disease
| |
| * Dermatitis herpetiformis – extraintestinal manifestation
| |
|
| |
|
| ==Diagnosis== | | ==Diagnosis== |
| There are several tests that can be used to assist in diagnosis. The level of symptoms may determine the order of the tests, but ''all'' tests lose their usefulness if the patient is already taking a gluten-free diet. Intestinal damage begins to heal within weeks of gluten being removed from the diet, and antibody levels decline over months. For those who have already started on a gluten-free diet, it may be necessary to perform a re-challenge with 10 g of gluten (four slices of bread) per day over 2–6 weeks before repeating the investigations. Those who experience severe symptoms (e.g. diarrhoea) earlier can be regarded as sufficiently challenged and can be tested earlier.<ref name=Ciclitira/>
| | [[Celiac disease history and symptoms|History and Symptoms]] | [[Celiac disease physical examination|Physical Examination]] | [[Celiac disease laboratory tests|Laboratory Findings]] | [[Celiac disease other imaging findings|Other Imaging Findings]] | [[Celiac disease other diagnostic studies|Other Diagnostic Studies]] |
| | |
| Combining findings into a prediction rule to guide use of endoscopy reported a [[sensitivity (tests)|sensitivity]] of 100% (it would identify all the cases) and [[specificity (tests)|specificity]] of 61% (it would be incorrectly positive in 39%). The prediction rule recommends that patients with high risk symptoms ''or'' positive serology should undergo endoscopy. The study defined high risk symptoms as weight loss, anaemia (haemoglobin less than 120 g/l in females and less than 130 g/l in males), or diarrhoea (more than three loose stools per day).<ref name="pmmid17383983">{{cite journal |author=Hopper A, Cross S, Hurlstone D, McAlindon M, Lobo A, Hadjivassiliou M, Sloan M, Dixon S, Sanders D |title=Pre-endoscopy serological testing for coeliac disease: evaluation of a clinical decision tool |journal=BMJ |volume=334 |issue= |pages=729 |year=2007 |pmid=17383983}}</ref>
| |
| | |
| == History and Symptoms ==
| |
| * May be asymptomatic
| |
| * Vague [[abdominal pain]]
| |
| * [[Diarrhea]]
| |
| * [[Weight loss]]
| |
| * [[Malabsorption]] / [[steatorrhea]]
| |
| *:* ''Symptoms and pathologic changes resolve with gluten-free diet''
| |
| | |
| In majority of cases diagnosis can be made reliably with blood testing, but the "gold standard" is still small bowel intestinal biopsy (obtained via EGD). Ideally, a small bowel biopsy should be taken while the patient is on a gluten diet and then checked against a follow-up biopsy after 12 weeks on a gluten-free diet. IgA anti-endomysial antibody (EmA) is at least 99% specific, and about 93% sensitive, but some studies show it to be nearly 100% specific and sensitive. Tissue anti-transglutaminase (tTG) antibody is similarly accurate. Total serum IgA is usually normal, but may be low to undetectable in a small percentage patients. If total IgA is very low, EmA and tTG will not be accurate and a small bowel biopsy should be considered. IgA anti-gliadin antibody (AGA) is more useful as a screening test, and neither anti-gliadin IgA or IgG antibodies are as specific or sensitive as anti-endomysial antibodies or anti-transglutaminase antibodies.
| |
| | |
| Classic symptoms of coeliac disease include [[diarrhoea]], [[weight loss]] (or stunted growth in children), and [[fatigue (physical)|fatigue]], but while coeliac disease is primarily a bowel disease, bowel symptoms may also be limited or even absent. Some patients are diagnosed with symptoms related to the decreased absorption of nutrients or with various symptoms which, although statistically linked, have no clear relationship with the malfunctioning bowel. Given this wide range of possible symptoms, the classic triad is no longer a requirement for diagnosis.
| |
| | |
| Children between 9 and 24 months tend to present with bowel symptoms and growth problems shortly after first exposure to gluten-containing products. Older children may have more malabsorption-related problems and psychosocial problems, while adults generally have malabsorptive problems.<ref name=Ciclitira>{{cite web | first = P | last = Ciclitira | year= 2002 | url = http://www.bsg.org.uk/bsgdisp1.php?id=c9c5177d2b91e3228066 | title = Interim Guidelines for the Management of Patients with Coeliac Disease | publisher = British Society of Gastroenterology | accessdate = 2007-03-07}}</ref> Many adults with subtle disease only have fatigue or [[anaemia]].<ref name=VanHeelWest/>
| |
| | |
| * Behavioural changes
| |
| * Fatigue, malaise
| |
| * Growth delay
| |
| * weight loss (explained and unexplained)
| |
| | |
| ===Hematological===
| |
| * [[Anemia]]
| |
| * Hematologic diathesis
| |
| | |
| ===Skin/Mucous Membrane===
| |
| * Dermatitis herpetiformis
| |
| * Alopecia (both universalis and areata)
| |
| * Aphthous ulcers
| |
| * Abdominal or generalized swelling
| |
| * Epistaxsis
| |
| * Easy buisability
| |
| * Cheliosis, stomatitis
| |
| * Scaly dermatitis
| |
| | |
| ===Musculoskeletal===
| |
| * Non-specific bone and/or joint pain
| |
| * Osteopenia
| |
| * Tetany
| |
| | |
| ===Neurological===
| |
| * Peripheral neuropathy
| |
| * Seizures
| |
| | |
| ===Gastrointestinal===
| |
| The [[diarrhoea]] characteristic of coeliac disease is [[steatorrhoea|pale]], voluminous and malodorous. [[Abdominal pain]] and cramping, bloatedness with abdominal distention (thought to be due to fermentative production of bowel gas) and [[mouth ulcer]]s<ref>{{cite journal | author = Ferguson R, Basu M, Asquith P, Cooke W | title = Jejunal mucosal abnormalities in patients with recurrent aphthous ulceration | journal = Br Med J | volume = 1 | issue = 6000 | pages = 11–13 | year = 1976|id = PMID 1247715}}</ref> may be present. As the bowel becomes more damaged, a degree of [[lactose intolerance]] may develop. However, the variety of gastrointestinal symptoms that may be present in patients with coeliac disease is great, and some may have a normal bowel habit or even tend towards [[constipation]]. Frequently the symptoms are ascribed to [[irritable bowel syndrome]] (IBS), only later to be recognised as coeliac disease; a small proportion of patients with symptoms of IBS have underlying coeliac disease, and screening may be justified.<ref name=Spiegel>{{cite journal | author = Spiegel BM, DeRosa VP, Gralnek IM, Wang V, Dulai GS | year = 2004 | month = Jun | title = Testing for celiac sprue in irritable bowel syndrome with predominant diarrhea: a cost-effectiveness analysis | journal = Gastroenterology | volume = 126 | issue = 7 | pages = 1721–32 | id = PMID 15188167}}</ref>
| |
| | |
| Coeliac disease leads to an increased risk of both [[adenocarcinoma]] and [[lymphoma]] of the small bowel, which returns to baseline with diet. Longstanding disease may lead to other complications, such as ''ulcerative jejunitis'' (ulcer formation of the small bowel) and stricturing (narrowing as a result of scarring).<ref name=AGA>{{cite journal | author = | title = American Gastroenterological Association medical position statement: Celiac Sprue | journal = Gastroenterology | volume = 120 | issue = 6 | pages = 1522–5 | year = 2001 | id = PMID 11313323 | url = http://www.gastrojournal.org/article/PIIS0016508501701618/fulltext}}</ref>
| |
| | |
| As a summary:
| |
| * Abdominal pain
| |
| * [[Anorexia]]
| |
| * [[Diarrhea]]
| |
| * Flatulence, distention
| |
| * [[hepatic disease]]
| |
| * [[Hypoglycemia]]
| |
| * [[Malabsorbtion]]
| |
| * [[Steatorrhea]] and greasy, bulky stools
| |
| | |
| ===Malabsorption-related===
| |
| The changes in the bowel make it less able to absorb nutrients, minerals and the fat-soluble vitamins A, D, E, and K.<ref name=Ciclitira/>
| |
| * The inability to absorb carbohydrates and fats may cause [[weight loss]] (or [[failure to thrive]]/stunted growth in children) and [[fatigue (physical)|fatigue]] or lack of energy.
| |
| * [[Anaemia]] may develop in several ways: iron malabsorption may cause [[iron deficiency anemia|iron deficiency anaemia]], and [[folic acid]] and [[vitamin B12]] malabsorption may give rise to [[megaloblastic anemia|megaloblastic anaemia]].
| |
| * [[Calcium in biology|Calcium]] and [[vitamin D]] malabsorption (and compensatory secondary [[hyperparathyroidism]]) may cause [[osteopenia]] (decreased mineral content of the bone) or [[osteoporosis]] (bone weakening and risk of fragility fractures).
| |
| * A small proportion (10%) have abnormal [[coagulation]] due to deficiency of [[vitamin K]], and are slightly at risk for abnormal bleeding.
| |
| * Coeliac disease is also associated with [[small bowel bacterial overgrowth syndrome|bacterial overgrowth]] of the [[small intestine]], which can worsen malabsorption, or cause malabsorption after treatment.<ref name=Tursi>{{cite journal | author = Tursi A, Brandimarte G, Giorgetti G | title = High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal | journal = Am J Gastroenterol | volume = 98 | issue = 4 | pages = 839-43 | year = 2003 | id = PMID 12738465}}</ref>
| |
| | |
| ===Miscellaneous===
| |
| Coeliac disease has been linked with a number of conditions. In many cases it is unclear whether the gluten-induced bowel disease is a causative factor or whether these conditions share a common predisposition.
| |
| * [[IgA deficiency]] is present in 2% of patients with coeliac disease, and in turn this condition features a tenfold increased risk of coeliac disease.<ref>{{cite journal | author = Crabbé P, Heremans J | title = Selective IgA deficiency with steatorrhea. A new syndrome | journal = Am J Med | volume = 42 | issue = 2 | pages = 319-26 | year = 1967 | id = PMID 4959869}}</ref><ref>{{cite journal | author = Collin P, Mäki M, Keyriläinen O, Hällström O, Reunala T, Pasternack A | title = Selective IgA deficiency and coeliac disease | journal = Scand J Gastroenterol | volume = 27 | issue = 5 | pages = 367-71 | year = 1992|id = PMID 1529270}}</ref> Other features of this condition are an increased risk of [[infection]]s and [[autoimmune disease]].
| |
| * [[Dermatitis herpetiformis]]; this itchy cutaneous condition has been linked to a transglutaminase enzyme in the skin, features small bowel changes identical to those in coeliac disease<ref name=Marks>{{cite journal | author = Marks J, Shuster S, Watson A | title = Small-bowel changes in dermatitis herpetiformis | journal = Lancet | volume = 2 | issue = 7476 | pages = 1280–2 | year = 1966 | id = PMID 4163419}}</ref> and occurs more often (in 2%) in patients with coeliac disease.<ref name=Ciclitira/>
| |
| * Neurological associations: [[epilepsy]], [[ataxia]] (coordination problems), [[myelopathy]] and [[peripheral neuropathy]] have all been linked with coeliac disease, but the strength of these associations and the causality is still subject of debate.<ref>{{cite journal | author = Pengiran Tengah D, Wills A, Holmes G | title = Neurological complications of coeliac disease | journal = Postgrad Med J | volume = 78 | issue = 921 | pages = 393-8 | year = 2002 | url = http://pmj.bmjjournals.com/cgi/content/full/78/921/393 | id = PMID 12151653}}</ref>
| |
| * [[Growth failure]] and/or [[delayed puberty|pubertal delay]] in later childhood can occur even without obvious bowel symptoms or severe [[malnutrition]]. Evaluation of growth failure often includes coeliac screening.
| |
| * [[Miscarriage]] and [[infertility]].
| |
| * [[Hyposplenism]] (a small and underactive [[spleen]]) - it is unclear whether this actually increases infection risk in the same way as in other people without a functioning spleen.<ref name=Ferguson>{{cite journal | author = Ferguson A, Hutton M, Maxwell J, Murray D | title = Adult coeliac disease in hyposplenic patients | journal = Lancet | volume = 1 | issue = 7639 | pages = 163-4 | year = 1970 | id = PMID 4189238}}</ref>
| |
| * Other auto-immune disorders: [[diabetes mellitus type 1]],<ref name=Holmes>{{cite journal |author=Holmes G |title=Coeliac disease and Type 1 diabetes mellitus - the case for screening |journal=Diabet Med |volume=18 |issue=3 |pages=169-77 |year=2001 |pmid=11318836}}</ref> [[thyroiditis|autoimmune thyroiditis]],<ref>{{cite journal | author = Collin P, Kaukinen K, Välimäki M, Salmi J | title = Endocrinological disorders and celiac disease | journal = Endocr Rev | volume = 23 | issue = 4 | pages = 464-83 | year = 2002 | url = http://edrv.endojournals.org/cgi/content/full/23/4/464 | id = PMID 12202461}}</ref> [[primary biliary cirrhosis]]<!--
| |
| --><ref>{{cite journal | author = Kingham J, Parker D | title = The association between primary biliary cirrhosis and coeliac disease: a study of relative prevalences | journal = Gut | volume = 42 | issue = 1 | pages = 120-2 | year = 1998|id = PMID 9518232}}</ref> <!--
| |
| -->and [[microscopic colitis]].<ref>{{cite journal | author = Matteoni C, Goldblum J, Wang N, Brzezinski A, Achkar E, Soffer E | title = Celiac disease is highly prevalent in lymphocytic colitis | journal = J Clin Gastroenterol | volume = 32 | issue = 3 | pages = 225-7 | year = 2001 | id = PMID 11246349}}</ref>
| |
| | |
| ===Role of other grains===
| |
| Wheat varieties or subspecies containing gluten such as spelt and Kamut®, and the rye/wheat hybrid triticale, also trigger symptoms.<ref name="Grain toxicity">{{cite web | title = Grain toxicity | publisher = The CELIAC list|url = http://www.enabling.org/ia/celiac/doc/grains.rtf
| |
| | format = [[Rich Text Format|RTF]] | accessdate = 2006-08-27}}</ref>
| |
| | |
| Barley and rye also induce symptoms of coeliac disease.<ref name="Grain toxicity"/> A small minority of coeliac patients also react to oats.<ref>{{cite journal | author = Lundin K, Nilsen E, Scott H, Løberg E, Gjøen A, Bratlie J, Skar V, Mendez E, Løvik A, Kett K | title = Oats induced villous atrophy in coeliac disease | journal = Gut | volume = 52 | issue = 11 | pages = 1649–52 | year = 2003 | url = http://gut.bmjjournals.com/cgi/content/full/52/11/1649 | id = PMID 14570737}}</ref><ref>{{cite journal | author = Størsrud S, Olsson M, Arvidsson Lenner R, Nilsson L, Nilsson O, Kilander A | title = Adult coeliac patients do tolerate large amounts of oats | journal = Eur J Clin Nutr | volume = 57 | issue = 1 | pages = 163-9 | year = 2003 | id = PMID 12548312|url= http://www.nature.com/ejcn/journal/v57/n1/abs/1601525a.html}}</ref> Most probably oats produce symptoms due to cross contamination with other grains in the fields or in the distribution channels.<ref name=Ciclitira/> There is at least one oat vendor, Gluten Free Oats®, that offers oats that can be considered SAFE for people who are gluten intolerant because they are tested to be below 10 parts per million (ppm) by the University of Nebraska FARRP Laboratory <ref>http://www.glutenfreeoats.com, http://www.farrp.org/, http://www.farrp.org/analysis.htm</ref>. Another vendor (McCann's) which, while not claiming to be gluten-free, points out that the risk of contamination from their Oats product is low due to the processes they use.<ref>{{cite web |url=http://www.mccanns.ie/pages/faq.html |title=McCann's FAQ |accessdate=2006-11-03 |year=2004 |publisher=Odlum Group |quote=we reckon that the level of non-oat grains to be less than 0.05% }}</ref> Other cereals, such as maize (corn), quinoa, millet, sorghum, rice are safe for a patient to consume. Other carbohydrate-rich foods such as potatoes and bananas do not contain gluten and do not trigger symptoms.
| |
| | |
| *Sumarizing Lab Tests for Celiac Disease
| |
| **AGA is more useful as a screening test
| |
| **EmA and tTG are very sensitive and specific
| |
| **EmA and tTG will not be accurate if total serum IgA is low
| |
| **Small bowel biopsy is still the "gold standard"
| |
| | |
| ===Blood tests===
| |
| | |
| ==== Electrolyte and Biomarker Studies ====
| |
| * Complete blood count (CBC)
| |
| * Liver function tests (LFTs)
| |
| * B12
| |
| * Folate
| |
| * Ferritin (+/- Ca, alk phos) – at diagnosis and annually
| |
| | |
| ====Antibody testing====
| |
| [[Serology]] by [[blood test]] is useful both in diagnosing coeliac disease (high sensitivity of about 98%, i.e. it misses 2 in 100 cases) and in excluding it (high [[Specificity (tests)|specificity]] of over 95%, i.e. a positive test is most likely confirmative of coeliac disease rather than another condition). Because of the major implications of a diagnosis of coeliac disease, professional guidelines recommend that a positive blood test is still followed by an endoscopy. A negative test may still prompt a biopsy if the suspicion remains very high; this would pick up the remaining 2% undiagnosed cases, as well as offering alternative explanations for the symptoms. As such, endoscopy with biopsy is still considered the [[gold standard (test)|gold standard]] in the diagnosis of coeliac disease.<ref name=Ciclitira/><ref name=AGA/>
| |
| | |
| {| class="wikitable" align="left" style="margin-right:2em"
| |
| |+ Blood antibody tests for coeliac disease<ref name="pmid17785484"/>
| |
| |-
| |
| ! width="110px" | Test !! width="90px" | [[sensitivity (tests)|sensitivity]] || width="90px" | [[specificity (tests)|specificity]]
| |
| |-
| |
| | [[Anti-gliadin antibodies |AGA]] [[IgA]] || align="Center" | 50% || align="Center" | 98%
| |
| |-
| |
| | [[Anti-gliadin antibodies |AGA]] [[IgA]] || align="Center" | 25% || align="Center" | 98%
| |
| |-
| |
| | Anti-[[endomysium|EMA]] || align="Center" | 81% || align="Center" | 99%
| |
| |-
| |
| | [[Anti-transglutaminase antibodies |ATA]] (Anti-[[Tissue transglutaminase|TTG]]) || align="Center" | 81% || align="Center" | 99%
| |
| |}
| |
| | |
| Four serological blood tests exist for coeliac disease. The most widely used ones detect an [[antibody]] of the [[IgA]] type against particular [[antigen]]s in the small bowel. Older tests detected antibodies against [[reticulin]] (ARA) or [[gliadin]] ([[Anti-gliadin antibodies |AGA]]), but recent evidence supports the use of the more modern tests, namely those detecting IgA antibodies against [[endomysium]] (EMA) or [[tissue transglutaminase]] (TTG). Generally, serology may be unreliable in young children, with anti-gliadin performing somewhat better than other tests in children under five.<ref name=HillNIH>Hill ID. "What are the sensitivity and specificity of serological tests for celiac disease? Do sensitivity and specificity vary in different populations?" In: ''NIH Consensus Development Conference on Celiac Disease''. Bethesda, Md.: U.S. National Institutes of Health, 2004;27–31. [http://consensus.nih.gov/2004/2004CeliacDisease118Program.pdf PDF].</ref> Serology tests are based on [[immunofluorescence|indirect immunofluorescence]] (reticulin, gliadin and endomysium) or [[ELISA]] (gliadin or tissue transglutaminase).<ref>{{cite journal |author=Wong R, Steele R, Reeves G, Wilson R, Pink A, Adelstein S |title=Antibody and genetic testing in coeliac disease |journal=Pathology |volume=35 |issue=4 |pages=285–304 |year=2003 |pmid=12959764}}</ref>
| |
| | |
| Guidelines recommend that a total serum IgA level is checked in parallel, as coeliac patients with IgA deficiency may be unable to produce the antibodies on which these tests depend ("[[Type I and type II errors|false negative]]"). In those patients, IgG antibodies against transglutaminase (IgG-TTG) may be diagnostic.<ref>{{cite journal | author = Korponay-Szabó I, Dahlbom I, Laurila K, Koskinen S, Woolley N, Partanen J, Kovács J, Mäki M, Hansson T | title = Elevation of IgG antibodies against tissue transglutaminase as a diagnostic tool for coeliac disease in selective IgA deficiency | journal = Gut | volume = 52 | issue = 11 | pages = 1567–71 | year = 2003 | url = http://gut.bmjjournals.com/cgi/content/full/52/11/1567 | id = PMID 14570724}}</ref>
| |
| {| class="wikitable" align="right"
| |
| |+ Blood HLA tests for coeliac disease<ref name="pmid17785484"/>
| |
| ! width="70px" | Test !! width="90px" | [[sensitivity (tests)|sensitivity]] || width="90px" | [[specificity (tests)|specificity]]
| |
| |-
| |
| | [[HLA-DQ2]] || align="center" | 94% || align="center" | 73%
| |
| |-
| |
| | [[HLA-DQ8]] || align="center" | 12% || align="center" | 81%
| |
| |}
| |
| | |
| ====HLA genetic typing====
| |
| Antibody testing and [[Human leukocyte antigen|HLA]] testing have similar accuracies.<ref name="pmid17785484"/>
| |
| <br/>
| |
| | |
| <br/>
| |
| <br/>
| |
| | |
| ===Endoscopy===
| |
| [[Image:celiac_3.jpg|left|thumb|200px|[[Endoscopy|Endoscopic]] still of [[duodenum]] of patient with coeliac disease showing scalloping of folds.]]
| |
| An [[upper endoscopy]] with [[biopsy]] of the [[duodenum]] (beyond the [[duodenal bulb]]) or [[jejunum]] is performed. It is important for the physician to obtain multiple samples (four to eight) from the duodenum. Not all areas may be equally affected; if biopsies are taken from healthy bowel, it would result in false negative results.<ref name=AGA/>
| |
| | |
| Most patients with coeliac disease have a small bowel that appears normal on endoscopy; however, five endoscopic findings have been associated with a high specificity for coeliac disease when all are found: scalloping of the small bowel folds (''pictured''), paucity in the folds, a mosaic pattern to the [[mucosa]] (described as a ''cracked-mud'' appearance), prominence of the submucosal blood vessels and a nodular pattern to the mucosa.<ref>{{cite journal | author = Niveloni S, Fiorini A, Dezi R, Pedreira S, Smecuol E, Vazquez H, Cabanne A, Boerr LA, Valero J, Kogan Z, Maurino E, Bai JC. | title = Usefulness of videoduodenoscopy and vital dye staining as indicators of mucosal atrophy of celiac disease: assessment of interobserver agreement | journal = Gastrointestinal Endoscopy | volume = 47 | issue = 3 | pages = 223–229 | year = 1998 | id = PMID 9580349}}</ref>
| |
| | |
| Until the 1970s, biopsies were obtained using metal capsules attached to a suction device. The capsule was swallowed and allowed to pass into the small intestine. After X-ray verification of its position, suction was applied to collect part of the intestinal wall inside the capsule. One much utilized capsule system is the [[Watson capsule]]. This method has now been largely replaced by fiberoptic endoscopy, which carries a higher sensitivity rate and a lower error frequency.<ref>{{cite journal |author=Mee A, Burke M, Vallon A, Newman J, Cotton P |title=Small bowel biopsy for malabsorption: comparison of the diagnostic adequacy of endoscopic forceps and capsule biopsy specimens |journal=Br Med J (Clin Res Ed) |volume=291 |issue=6498 |pages=769-72 |year=1985 |pmid=3929934}}</ref>
| |
| | |
| ===Pathology===
| |
| The classic pathology changes of coeliac disease in the small bowel are categorized by the "Marsh classification":<ref>{{cite journal | author = Marsh M | title = Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue') | journal = Gastroenterology | volume = 102 | issue = 1 | pages = 330-54 | year = 1992 | id = PMID 1727768}}</ref>
| |
| *Marsh stage 0: normal mucosa
| |
| *Marsh stage 1: increased number of intra-epithelial [[lymphocytes]], usually exceeding 20 per 100 [[enterocyte]]s
| |
| *Marsh stage 2: proliferation of the [[crypts of Lieberkuhn]]
| |
| *Marsh stage 3: partial or complete [[intestinal villus|villous]] [[atrophy]]
| |
| *Marsh stage 4: [[hypoplasia]] of the [[small bowel]] architecture
| |
| | |
| The changes classically improve or reverse after [[gluten]] is removed from the diet, so many official guidelines recommend a repeat [[biopsy]] several (4–6) months after commencement of gluten exclusion.<ref name=Ciclitira/>
| |
| | |
| In some cases a deliberate gluten challenge, followed by biopsy, may be conducted to confirm or refute the diagnosis. A normal biopsy and normal serology after challenge indicates the diagnosis may have been incorrect.<ref name=Ciclitira/> Patients are warned that one does not "outgrow" coeliac disease in the same way as childhood food intolerances.
| |
| [[Image:CoeliacDisease.png|left|thumb|300px|Schematic of the Marsh classification of upper [[jejunum|jejunal]] pathology in coeliac disease]]
| |
| | |
| ===Other diagnostic tests===
| |
| Other tests that may assist in the diagnosis are [[blood test]]s for a [[full blood count]], [[electrolyte]]s, [[calcium]], [[renal function]], [[liver enzyme]]s, [[vitamin B12]] and [[folic acid]] levels. [[Coagulation]] testing ([[prothrombin time]] and [[partial thromboplastin time]]) may be useful to identify deficiency of [[vitamin K]], which predisposes patients to [[hemorrhage]]. These tests should be repeated on follow-up, as well as [[anti-transglutaminase antibodies|anti-tTG]] titres.<ref name=Ciclitira/> | |
| | |
| Some professional guidelines<ref name=Ciclitira/> recommend screening of all patients for [[osteoporosis]] by [[dual energy X-ray absorptiometry|DXA/DEXA]] scanning.
| |
| | |
| ===Genetics===
| |
| The vast majority of coeliac patients have one of two types of [[HLA DQ]].<ref name="pmid17785484">{{cite journal |author=Hadithi M, von Blomberg BM, Crusius JB, ''et al'' |title=Accuracy of serologic tests and HLA-DQ typing for diagnosing celiac disease |journal=Ann. Intern. Med. |volume=147 |issue=5 |pages=294–302 |year=2007 |pmid=17785484 |doi=|url=http://www.annals.org/cgi/content/full/147/5/294}}</ref> This [[gene]] is part of the [[major histocompatibility complex|MHC class II antigen-presenting receptor]] (also called the [[human leukocyte antigen]]) system and distinguishes cells between self and non-self for the purposes of the [[immune system]]. There are 7 HLA DQ variants (DQ2 and DQ4 through 9). Two of these variants—[[HLA-DQ2|DQ2]] and [[HLA-DQ8|DQ8]]—are associated with coeliac disease. The gene is located on the short arm of the [[Chromosome 6 (human)|sixth chromosome]], and as a result of the [[genetic linkage|linkage]] this [[locus (genetics)|locus]] has been labeled CELIAC1.
| |
| | |
| Over 95% of coeliac patients have an isoform of DQ2 (encoded by DQA1*05 and DQB1*02 genes) and [[HLA-DQ8|DQ8]] (encoded by the [[haplotype]] DQA1*03:DQB1*0302), which is inherited in families. The reason these genes produce an increase in risk of coeliac disease is that the receptors formed by these genes bind to [[gliadin]] peptides more tightly than other forms of the antigen-presenting receptor. Therefore, these forms of the receptor are more likely to activate [[T cell|T lymphocytes]] and initiate the autoimmune process.<ref name=VanHeelWest/>
| |
| | |
| [[Image:DQa2b5_da_gliadin.jpg|frame|left|DQ α<sup>5</sup>-β<sup>2</sup> -binding cleft with a deamidated gliadin peptide (yellow), modified from {{PDB|1S9V}}<ref>{{cite journal | author = Kim C, Quarsten H, Bergseng E, Khosla C, Sollid L | title = Structural basis for HLA-DQ2-mediated presentation of gluten epitopes in celiac disease | journal = Proc Natl Acad Sci U S A | volume = 101 | issue = 12 | pages = 4175–9 | year = 2004 | id = PMID 15020763}}</ref> ]]
| |
| | |
| Most coeliac patients bear a two-gene [[HLA-DQ]] [[haplotype]] referred to as [[HLA-DQ2#DQ2.5|DQ2.5 haplotype]]. This haplotype is composed of 2 adjacent gene [[allele]]s, DQA1*0501 and [[HLA-DQ2#DQB1.2A0201|DQB1*0201]], which encode the two subunits, DQ α<sup>5</sup> and DQ β<sup>2</sup>. In most individuals, this DQ2.5 isoform is encoded by one of two [[Chromosome 6 (human)|chromosomes 6]] inherited from parents. Most coeliacs inherit only one copy of this DQ2.5 haplotype, while some inherit it from ''both'' parents; the latter are especially at risk for coeliac disease, as well as being more susceptible to severe complications.<ref name="pmid17190762">{{cite journal | author = Jores RD, Frau F, Cucca F, ''et al'' | title = HLA-DQB1*0201 homozygosis predisposes to severe intestinal damage in celiac disease | journal = Scand. J. Gastroenterol. | volume = 42 | issue = 1 | pages = 48-53 | year = 2007 | pmid = 17190762 | doi = 10.1080/00365520600789859}}</ref> Some individuals inherit DQ2.5 from one parent and portions of the haplotype ([[HLA-DQ|DQB1*02]] or DQA1*05) from the other parent, increasing risk. Less commonly, some individuals inherit the DQA1*05 allele from one parent and the DQB1*02 from the other parent, called a trans-haplotype association, and these individuals are at similar risk for coeliac disease as those with a single DQ2.5 bearing chromosome 6, but in this instance disease tends not to be familial. Among the 6% of European celiacs that do not have DQ2.5(cis or trans) or DQ8, 4% are DQ2 and 2% DQA1*05, 0.4% cannot be linked to DQ8, DQA1*05, or DQB1*02.<ref name="pmid12651074">{{cite journal |author=Karell K, Louka AS, Moodie SJ, ''et al'' |title=HLA types in celiac disease patients not carrying the DQA1*05-DQB1*02 (DQ2) heterodimer: results from the European Genetics Cluster on Celiac Disease |journal=Hum. Immunol. |volume=64 |issue=4 |pages=469-77 |year=2003 |pmid=12651074 |doi= |issn=}}</ref>
| |
| | |
| The frequency of these genes varies geographically. DQ2.5 has high frequency in peoples of North and Western Europe (Basque Country, Ireland,<ref>{{cite journal | author = Michalski J, McCombs C, Arai T, Elston R, Cao T, McCarthy C, Stevens F | title = HLA-DR, DQ genotypes of celiac disease patients and healthy subjects from the West of Ireland | journal = Tissue Antigens | volume = 47 | issue = 2 | pages = 127-33 | year = 1996 | id = PMID 8851726}}</ref> with highest frequencies), portions of Africa, and is associated disease in India,<ref>{{cite journal |author=Kaur G, Sarkar N, Bhatnagar S, ''et al'' |title=Pediatric celiac disease in India is associated with multiple DR3-DQ2 haplotypes |journal=Hum. Immunol. |volume=63 |issue=8 |pages=677-82 |year=2002 |pmid=12121676 |doi=}}</ref> but is not found along portions of the West Pacific rim. DQ8, spread more globally than DQ2.5, is more prevalent from South and Central America (up to 90% [[phenotype]] frequency).<ref>{{cite journal | author = Layrisse Z, Guedez Y, Domínguez E, Paz N, Montagnani S, Matos M, Herrera F, Ogando V, Balbas O, Rodríguez-Larralde A | title = Extended HLA haplotypes in a Carib Amerindian population: the Yucpa of the Perija Range | journal = Hum Immunol | volume = 62 | issue = 9 | pages = 992–1000 | year = 2001 | id = PMID 11543901}}</ref>
| |
| | |
| In addition to the CELIAC1 locus, CELIAC2 ([[Chromosome 5 (human)|5]]q31-q33 - IBD5 locus), CELIAC3 ([[Chromosome 2 (human)|2]]q33 -CTLA4 locus), CELIAC4 ([[Chromosome 19 (human)|19]]q13.1 - MYOIXB locus), have been linked to coeliac disease. The [[CTLA4]] and [[myosin IXB]] and gene have been found to be linked to coeliac disease and other autoimmune diseases.<ref name="pmid16025348">{{cite journal | author = Zhernakova A, Eerligh P, Barrera P, ''et al'' | title = CTLA4 is differentially associated with autoimmune diseases in the Dutch population | journal = Hum. Genet. | volume = 118 | issue = 1 | pages = 58-66 | year = 2005 | pmid = 16025348 | doi = 10.1007/s00439-005-0006-z}}</ref><ref name="pmid17584584">{{cite journal | author = Sánchez E, Alizadeh BZ, Valdigem G, ''et al'' | title = MYO9B gene polymorphisms are associated with autoimmune diseases in Spanish population | journal = Hum. Immunol. | volume = 68 | issue = 7 | pages = 610-5 | year = 2007 | pmid = 17584584 | doi = 10.1016/j.humimm.2007.03.006}}</ref> Two additional loci on [[Chromosome 4 (human)|chromosome 4]], 4q27 (IL2 or IL21 locus) and 4q14, have been found to be linked to coeliac disease.<ref name="pmid17558408">{{cite journal | author = van Heel DA, Franke L, Hunt KA, ''et al'' | title = A genome-wide association study for celiac disease identifies risk variants in the region harboring IL2 and IL21 | journal = Nat Genet | volume = 39 | issue = 7 | pages = 827-9 | year = 2007 | pmid = 17558408 | doi = 10.1038/ng2058}}</ref><ref>{{cite journal | author = Popat S, Bevan S, Braegger C, Busch A, O'Donoghue D, Falth-Magnusson K, Godkin A, Hogberg L, Holmes G, Hosie K, Howdle P, Jenkins H, Jewell D, Johnston S, Kennedy N, Kumar P, Logan R, Love A, Marsh M, Mulder C, Sjoberg K, Stenhammar L, Walker-Smith J, Houlston R | title = Genome screening of coeliac disease | journal = J Med Genet | volume = 39 | issue = 5 | pages = 328-31 | year = 2002 | url = http://jmg.bmjjournals.com/cgi/content/full/39/5/328 | id = PMID 12011149}}</ref>
| |
| | |
| ===Prolamins===
| |
| The proteins in food responsible for the immune reaction in coeliac disease are the [[prolamin]]s. These are storage proteins rich in [[proline]] (''prol-'') and glutamine- (-''amin'') that dissolve in alcohols and are resistant to [[pepsin]] and [[chymotrypsin]], the two main digestive [[protease]]s in the gut. Gliadin in wheat is the best-understood member of this family, but other prolamins exist and [[hordein]] (from barley), and [[secalin]] (from rye) may contribute to coeliac disease.<ref name=VanHeelWest/> However, not all prolaminins will cause this immune reaction and there is ongoing controversy on the ability of avenin (the prolamin found in oats) to induce this response in coeliac disease.
| |
| | |
| ===Tissue transglutaminase===
| |
| [[Image:Tissue transglutaminase.png|thumb|left|150px|[[Tissue transglutaminase]], drawn from {{PDB|1FAU}}.]]
| |
| [[Anti-transglutaminase antibodies]] to the enzyme [[tissue transglutaminase]] (tTG) are found in an overwhelming majority of cases.<ref name=Dieterich>{{cite journal | author = Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken E, Schuppan D | title = Identification of tissue transglutaminase as the autoantigen of celiac disease | journal = Nat Med | volume = 3 | issue = 7 | pages = 797–801 | year = 1997 | id = PMID 9212111}}</ref> Tissue transglutaminase modifies gluten [[peptide]]s into a form that may stimulate the immune system more effectively.<ref name=VanHeelWest/>
| |
| | |
| Stored biopsies from suspected coeliac patients has revealed that [[autoantibody]] deposits in the [[Wiktionary:subclinical|subclinical]] coeliacs are detected prior to clinical disease. These deposits are also found in patients who present with other autoimmune diseases, anemia or malabsorption phenomena at a much increased rate over the normal population.<ref name=Kaukinen>{{cite journal | author = Kaukinen K, Peraaho M, Collin P, Partanen J, Woolley N, Kaartinen T, Nuuntinen T, Halttunen T, Maki M, Korponay-Szabo I | title = Small-bowel mucosal tranglutaminase 2-specific IgA deposits in coeliac disease without villous atrophy: A Prospective and radmonized clinical study | journal = Scand J Gastroenterology | volume = 40 | pages = 564–572 | year = 2005 | id = PMID 16036509}}</ref> Endomysial component of antibodies (EMA) to tTG are believed to be directed toward cell surface transglutaminase, and these antibodies are still used in confirming a coeliac disease diagnosis. However, a 2006 study showed that EMA-negative coeliac patients tend to be older males with more severe abdominal symptoms and a lower frequency of "atypical" symptoms including autoimmune disease.<ref name=EMAnegCD>{{cite journal |author=Salmi T, Collin P, Korponay-Szabó I, Laurila K, Partanen J, Huhtala H, Király R, Lorand L, Reunala T, Mäki M, Kaukinen K |title=Endomysial antibody-negative coeliac disease: clinical characteristics and intestinal autoantibody deposits |journal=Gut |volume=55 |issue=12 |pages=1746–53 |year=2006 |pmid=16571636}}</ref> In this study the anti-tTG antibody deposits did not correlate with the severity of villous destruction. These findings, coupled with recent work showing that gliadin has an innate response component,<ref name=InnateReview>{{cite journal | author = Londei M, Ciacci C, Ricciardelli I, Vacca L, Quaratino S, and Maiuri L. | title = Gliadin as a stimulator of innate responses in celiac disease | journal = Mol Immunol | volume = 42 | issue = 8 | pages = 913–918 | year = 2005 | id = PMID 15829281}}</ref> suggests that gliadin may be more responsible for the primary manifestations of coeliac disease whereas tTG is a bigger factor in secondary effects such as allegic responses and secondary autoimmune diseases. In a large percentage of coeliac patients the anti-tTG antibodies also recognize a [[rotavirus]] protein called VP7. These antibodies stimulate monocytes proliferation and rotavirus infection might explain some early steps in the cascade of immune cell proliferation.<ref name = "toll-like">{{cite journal |author=Zanoni G, Navone R, Lunardi C, Tridente G, Bason C, Sivori S, Beri R, Dolcino M, Valletta E, Corrocher R, Puccetti A |title=In celiac disease, a subset of autoantibodies against transglutaminase binds toll-like receptor 4 and induces activation of monocytes |journal=PLoS Med |volume=3 |issue=9 |pages=e358 |year=2006 |pmid=16984219}}</ref> Indeed, earlier studies of rotavirus damage in the gut showed this causes a villous atrophy.<ref>{{cite journal | author = Salim A, Phillips A, Farthing M | title = Pathogenesis of gut virus infection | journal = Baillieres Clin Gastroenterol | volume = 4 | issue = 3 | pages = 593–607 | year = 1990 | id = PMID 1962725}}</ref> This suggests that viral proteins may take part in the initial flattening and stimulate self-crossreactive anti-VP7 production. Antibodies to VP7 may also slow healing until the gliadin mediated tTG presentation provides a second source of crossreactive antibodies.
| |
| | |
| ===Villous atrophy and malabsorption===
| |
| The inflammatory process, mediated by [[T cell]]s, leads to disruption of the structure and function of the small bowel's mucous lining, and causes [[malabsorption]] as it impairs the body's ability to absorb [[nutrient]]s, minerals and fat-soluble [[vitamin]]s A, D, E and K from food. [[Lactose intolerance]] may be present due to the decreased bowel surface and reduced production of [[lactase]] but typically resolves once the condition is treated.
| |
| | |
| Alternative causes of this tissue damage have been proposed and involve release of [[interleukin 15]] and activation of the innate immune system by a shorter gluten peptide (p31–43/49). This would trigger killing of [[enterocyte]]s by lymphocytes in the epithelium.<ref name=VanHeelWest/> The villous atrophy seen on biopsy may also be due to unrelated causes, such as [[tropical sprue]], [[giardiasis]] and [[radiation enteritis]]. While positive serology and typical biopsy are highly suggestive of coeliac disease, lack of response to diet may require these alternative diagnoses to be considered.<ref name=AGA/>
| |
| | |
| ===Risk modifiers===
| |
| There are various theories as to what determines whether a genetically susceptible individual will go on to develop coeliac disease. Major theories include infection by [[rotavirus]]<ref>{{cite journal |author=Stene L, Honeyman M, Hoffenberg E, Haas J, Sokol R, Emery L, Taki I, Norris J, Erlich H, Eisenbarth G, Rewers M |title=Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study |journal=Am J Gastroenterol |volume=101 |issue=10 |pages=2333–40 |year=2006 |pmid=17032199}}</ref> or human intestinal [[adenovirus]].<ref>{{cite journal | author = Kagnoff M, Paterson Y, Kumar P, Kasarda D, Carbone F, Unsworth D, Austin R | title = Evidence for the role of a human intestinal adenovirus in the pathogenesis of coeliac disease | journal = Gut | volume = 28 | issue = 8 | pages = 995–1001 | year = 1987 | id = PMID 2822550}}</ref> Some research has suggested that smoking is protective against adult onset coeliac disease.<ref>{{cite journal | author = Suman S, Williams E, Thomas P, Surgenor S, Snook J | title = Is the risk of adult coeliac disease causally related to cigarette exposure? | journal = Eur J Gastroenterol Hepatol | volume = 15 | issue = 9 | pages = 995–1000 | year = 2003 | id = PMID 12923372}}</ref>
| |
| | |
| A 2005 prospective and observational study found that timing of the exposure to gluten in childhood was an important risk modifier. People exposed to wheat, barley, or rye before the [[Gut flora|gut barrier]] has fully developed (three months after birth) had five times the risk of developing coeliac disease over those exposed at 4 to 6 months. Those exposed later had a slightly increased risk relative to those exposed at 4 to 6 months.<ref name="Norris">{{cite journal|author= Norris JM, Barriga K, Hoffenberg EJ, Taki I, Miao D, Haas JE, Emery LM, Sokol RJ, Erlich HA, Eisenbarth GS, Rewers M.|title=Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease | journal=JAMA | year=2005| volume=293 | issue=19| pages=2343–2351 | id=PMID 15900004}}</ref> However a 2006 study with similar numbers found just the reverse, that early introduction of grains was protective.<ref>{{cite journal |author=Poole J, Barriga K, Leung D, Hoffman M, Eisenbarth G, Rewers M, Norris J |title=Timing of initial exposure to cereal grains and the risk of wheat allergy |journal=Pediatrics |volume=117 |issue=6 |pages=2175–82 |year=2006 |pmid=16740862}}</ref> Breastfeeding may also reduce risk. A [[meta-analysis]] indicates that prolonging [[breastfeeding]] until the introduction of gluten-containing grains into the diet was associated with a 52% reduced risk of developing coeliac disease in infancy; whether this persists into adulthood is not clear.<ref>{{cite journal |author=Akobeng A, Ramanan A, Buchan I, Heller R |title=Effect of breast feeding on risk of coeliac disease: a systematic review and meta-analysis of observational studies |journal=Arch Dis Child |volume=91 |issue=1 |pages=39–43 |year=2006 |pmid=16287899}}</ref>
| |
|
| |
|
| ==Treatment== | | ==Treatment== |
| | [[Celiac disease medical therapy|Medical Therapy]] | [[Celiac disease surgery|Surgery]] | [[Celiac disease primary prevention|Primary Prevention]] | [[Celiac disease social impact|Social Impact]] | [[Celiac disease future or investigational therapies|Future or Investigational Therapies]] |
|
| |
|
| * Nutrition referral--strict gluten-free diet
| | ==Case Studies== |
| * Vitamin/mineral supplements until deficiencies resolve with gluten-free diet
| | [[Celiac disease case study one|Case #1]] |
| | |
| ===Diet===
| |
| {{main|Gluten-free diet}}
| |
| | |
| Presently, the only effective treatment is a life-long [[gluten-free diet]].<ref>{{cite journal | author = Kupper C | title = Dietary guidelines and implementation for celiac disease | journal = Gastroenterology | volume = 128 | issue = 4 Suppl 1 | pages = S121-7 | year = 2005 | id = PMID 15825119}}</ref> No medication exists that will prevent damage, or prevent the body from attacking the gut when gluten is present. Strict adherence to the diet allows the intestines to heal, leading to resolution of all symptoms in the vast majority of cases and, depending on how soon the diet is begun, can also eliminate the heightened risk of osteoporosis and intestinal cancer.<ref>{{cite journal | author = Treem W | title = Emerging concepts in celiac disease | journal = Curr Opin Pediatr | volume = 16 | issue = 5 | pages = 552-9 | year = 2004|id = PMID 15367850}}</ref> [[Dietician]] input is generally requested to ensure the patient is aware which foods contain gluten, which foods are safe, and how to have a balanced diet despite the limitations. In many countries gluten-free products are available on [[Medical prescription|prescription]] and may be reimbursed by [[health insurance]] plans. More manufacturers are producing gluten-free products, some of which are almost indistinguishable from their gluten-containing counterparts.
| |
| | |
| The diet can be cumbersome; while young children can be kept compliant by their parents, teenagers may wish to hide their problem or rebel against the dietary restrictions, risking relapse. Many food products contain traces of gluten even if apparently wheat-free. Gluten-free products are usually more expensive and harder to find than common wheat-containing foods.
| |
| | |
| Even while on a diet, health-related quality of life (HRQOL) may be decreased in people with coeliac disease. Some have persisting digestive symptoms or [[dermatitis herpetiformis]], mouth ulcers, osteoporosis and fractures. Symptoms suggestive of [[irritable bowel syndrome]] may be present, and there is an increased rate of anxiety, fatigue, [[dyspepsia]] and musculoskeletal pain.<ref>{{cite journal | author = Häuser W, Gold J, Stein J, Caspary W, Stallmach A | title = Health-related quality of life in adult coeliac disease in Germany: results of a national survey | journal = Eur J Gastroenterol Hepatol | volume = 18 | issue = 7 | pages = 747-54 | year = 2006 | id = PMID 16772832}}</ref>
| |
| | |
| ===Refractory disease===
| |
| A tiny minority of patients suffer from refractory disease, which means they do not improve on a gluten-free diet. This may be because the disease has been present for so long that the intestines are no longer able to heal on diet alone, or because the patient is not adhering to the diet, or because the patient is consuming foods that are inadvertently contaminated with gluten. If alternative causes have been eliminated, [[glucocorticoid|steroids]] or [[Immunosuppressive drug|immunosuppressants]] (such as [[azathioprine]]) may be considered in this scenario.<ref name=AGA/>
| |
| | |
| ===Experimental treatments===
| |
| Various other approaches are being studied that would reduce the need of dieting. All are still under development, and are not expected to be available to the general public for a while:<ref name=VanHeelWest/>
| |
| * [[Genetic engineering|Genetically engineered]] wheat species, or wheat species that have been selectively bred to be minimally immunogenic. This, however, could interfere with the effects that gliadin has on the quality of dough.
| |
| * A combination of [[enzyme]]s ([[prolyl endopeptidase]] and a barley glutamine-specific [[cysteine endopeptidase]] (EP-B2)) that degrade the putative 33-mer peptide in the [[duodenum]]. This combination would enable coeliac disease patients to consume gluten-containing products.<ref>{{cite journal | author = Siegel M, Bethune M, Gass J, Ehren J, Xia J, Johannsen A, Stuge T, Gray G, Lee P, Khosla C | title = Rational design of combination enzyme therapy for celiac sprue | journal = Chem Biol | volume = 13 | issue = 6 | pages = 649-58 | year = 2006 | id = PMID 16793522}}</ref>
| |
| * Inhibition of [[zonulin]], an endogenous signaling protein linked to increased permeability of the bowel wall and hence increased presentation of gliadin to the immune system.<ref>{{cite journal | author = Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, Goldblum S | title = Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease | journal = Lancet | volume = 355 | issue = 9214 | pages = 1518–9 | year = 2000 | id = PMID 10801176}}</ref>
| |
| * Other treatments aimed at other well-understood steps in the pathogenesis of coeliac disease, such as the action of HLA-DQ2 or tissue transglutaminase and the MICA/NKG2D interaction that may be involved in the killing of [[enterocyte]]s (bowel lining cells).
| |
| | |
| ==Screening and case finding==
| |
| There is significant debate as to the benefits of [[Screening (medicine)|screening]]. Some studies suggest that early detection would decrease the risk of osteoporosis and anaemia. In contrast, a [[cohort study|cohort studied]] in Cambridge suggested that people with undetected coeliac disease had a beneficial risk profile for [[cardiovascular disease]] (less [[overweight]], lower [[cholesterol]] levels).<ref name=VanHeelWest/>
| |
| | |
| Due to its high sensitivity, [[serology]] has been proposed as a screening measure, because the presence of antibodies would detect previously undiagnosed cases of coeliac disease and prevent its complications in those patients. Serology may also be used to monitor adherence to diet: in those who still ingest gluten, antibody levels remain elevated.<ref name=Ciclitira/><ref name=AGA/>
| |
| | |
| Clinical scenarios in which screening may be justified include type 1 diabetes,<ref name=Holmes/> unexplained iron-deficiency anemia,<ref>{{cite journal |author=Corazza G, Valentini R, Andreani M, D'Anchino M, Leva M, Ginaldi L, De Feudis L, Quaglino D, Gasbarrini G |title=Subclinical coeliac disease is a frequent cause of iron-deficiency anaemia |journal=Scand J Gastroenterol |volume=30 |issue=2 |pages=153–6 |year=1995 |pmid=7732338}}</ref><ref>{{cite journal |author=Ransford R, Hayes M, Palmer M, Hall M |title=A controlled, prospective screening study of celiac disease presenting as iron deficiency anemia |journal=J Clin Gastroenterol |volume=35 |issue=3 |pages=228–33 |year=2002 |pmid=12192198}}</ref> [[Down's syndrome]], [[Turner's syndrome]], [[irritable bowel syndrome]],<ref name=Spiegel/> [[Lupus erythematosus|lupus]], and autoimmune thyroid disease.<ref>{{cite journal |author=Sjöberg K, Carlsson A |title=Screening for celiac disease can be justified in high-risk groups |journal=Lakartidningen |volume=101 |issue=48 |pages=3912, 3915–6, 3918–9 |year=2004 |pmid=15631226}}</ref>
| |
| | |
| === Serologic Markers ===
| |
| * Immunoglobin A (IgA) antiendomysial antibody (Ab) = best test
| |
| *:* Sensitivity 85-98%
| |
| *:* Specificity 99%
| |
| *:* Limitation: 2-3% of patients have IgA deficiency false negative
| |
| * IgA tissue transglutaminase Ab
| |
| *:* Sensitivity 90-98%
| |
| *:* Specificity 95-97%
| |
| *:* Limitation: not widely available
| |
| * IgA, IgG antigliadin Abs
| |
| *:* Sensitivity 80-90% (IgA), 75-85% (IgG)
| |
| *:* Specificity 85-95% (IgA), 75-90% (IgG)
| |
| | |
| ==Social and religious issues==
| |
| ===Roman Catholic position===
| |
| Roman Catholic doctrine states that for a valid Eucharist the bread must be made from wheat. In 2002, the Congregation for the Doctrine of the Faith approved German-made low-gluten hosts, which meet all of the Catholic Church's requirements, for use in Italy; although not entirely gluten-free, they were also approved by the Italian Celiac Association.<ref>{{cite web | author = Scott Adams |url = http://www.celiac.com/st_prod.html?p_prodid=696 | title = Bishops in Italy Approve a German-made Low Gluten Eucharistic Host | date = August 2, 2002 | publisher = Celiac.com}}</ref> Some Catholic coeliac sufferers have requested permission to use rice wafers; such petitions have always been denied.<ref>{{cite news | author = Associated Press |url = http://www.msnbc.msn.com/id/5762478/ |title = Girl with digestive disease denied Communion | work = MSNBC |publisher = Microsoft | date = December 8, 2004 |accessdate = 2006-05-30}}</ref> The issue is more complex for priests. Though a Catholic (lay or ordained) receiving under either form is considered to have received Christ "whole and entire", the priest, who is acting ''in persona Christi'', is required to receive under both species when offering Mass — not for the validity of his Communion, but for the fullness of the sacrifice of the Mass. On August 22, 1994, the Congregation for the Doctrine of the Faith apparently barred coeliacs from ordination, stating, "Given the centrality of the celebration of the Eucharist in the life of the priest, candidates for the priesthood who are affected by coeliac disease or suffer from alcoholism or similar conditions may not be admitted to holy orders." After considerable debate, the congregation softened the ruling on 24 July 2003 to "Given the centrality of the celebration of the Eucharist in the life of a priest, one must proceed with great caution before admitting to Holy Orders those candidates unable to ingest gluten or alcohol without serious harm."<ref>Ratzinger, Joseph (July 24, 2003). ''Prot. 89/78-174 98''. Congregation for the Doctrine of the Faith. Full text at: {{cite web | url = http://www.usccb.org/liturgy/innews/1103.shtml | title = The Use of Mustum and Low-Gluten Hosts at Mass | accessdate = 2007-03-07 | work = BCL Newsletter | month = November | year = 2003 | publisher = United States Conference of Catholic Bishops}}</ref>
| |
| | |
| As of January 2004, an extremely low-gluten host became available in the United States. The Benedictine Sisters of Perpetual Adoration in Clyde, MO, after ten years of perseverance, trial, and error, have produced a low-gluten host safe for celiacs and also approved by the Catholic Church for use at Mass. Each host is made and packaged in a dedicated wheat-free / gluten-free environment. The hosts are made separately by hand, unlike the common host which is stamped out of a long thin sheet of bread by a cutter. Therefore, each host is a slightly different size and shape. Most importantly, the finished hosts have been analyzed for gluten content. The gluten content of these hosts is reported as 0.01 %. In actuality, the gluten content is probably less than 0.01%. Sister Lynn, OSB, said that the result of the analysis of the finished host revealed "no gluten detected". The hosts are labeled as 0.01 % since the lowest limit of detection of this analysis was 0.01 %. In an article from the Catholic Review (February 15, 2004) Dr. Alessio Fasano was quoted as declaring these hosts "perfectly safe for celiac sufferers."
| |
| <ref>{{cite web |url=http://www.catholic.org/featured/headline.php?ID=1340 |title=Liturgy: Gluten-free Hosts |accessdate=2007-06-17 |last=McNamara |first= Father Edward |date=[[2004-09-15]] |year=|month= |format= |work= Catholic Online}}</ref>
| |
| | |
| ===Coeliacs and Passover===
| |
| The Jewish festival of Pesach (Passover) may present problems with its obligation to eat matzo. Matzo is normally made from wheat or other gluten-containing grains, so oat matzo is used. Many products prepared for Passover are free of wheat, barley, spelt, oats, and rye, as many Orthodox (especially Hasidic) Jews avoid non-matzo wheat products (''gebroks'') altogether. Potato starch is the primary starch used to replace the grains.<ref>{{cite web | url = http://oukosher.org/index.php/articles/single/gluten_intolerance_celiac_allergies_and_pesach/ | title = Gluten Intolerance, Celiac, Allergies And Pesach | accessdate = 2006-09-03 | author = Rabbi Avraham Juravel | publisher = Orthodox Union }}</ref>
| |
| | |
| == Primary Prevention ==
| |
| * +/- Pneumococcal vaccine (splenic atrophy)
| |
| | |
| ==References==
| |
| {{reflist|2}}
| |
|
| |
|
| ==External links== | | ==External links== |
| * [http://digestive.niddk.nih.gov/ddiseases/pubs/celiac/ National Digestive Diseases Clearinghouse] - page on coeliac disease | | * [http://digestive.niddk.nih.gov/ddiseases/pubs/celiac/ National Digestive Diseases Clearinghouse] - page on coeliac disease |
| * [http://www.coeliac.co.uk/ Coeliac UK] - leading UK charity
| |
| * [http://www.celiac.com Celiac.com] - U.S. resource
| |
| * [http://www.celiac.org/ Celiac Disease Foundation] - U.S.
| |
| * [http://www.csaceliacs.org Celiac Sprue Association] - U.S.
| |
| * [http://www.celiaccentral.org/ National Foundation for Celiac Awareness] - U.S.
| |
| * [http://www.celiac.nih.gov NIH Celiac Disease Awareness Campaign] | | * [http://www.celiac.nih.gov NIH Celiac Disease Awareness Campaign] |
| * Outcomes of 2004 [http://consensus.nih.gov/2004/2004CeliacDisease118html.htm consensus development conference], U.S. [[National Institutes of Health]] | | * Outcomes of 2004 [http://consensus.nih.gov/2004/2004CeliacDisease118html.htm consensus development conference], U.S. [[National Institutes of Health]] |
| * [http://www.farrp.org/analysis.htm determination of gluten contamination]
| |
| *{{dmoz|Health/Conditions_and_Diseases/Digestive_Disorders/Intestinal/Celiac_Disease/}}
| |
|
| |
|
| {{SIB}}
| |
| {{Gastroenterology}}
| |
|
| |
|
| {{featured article}} | | {{featured article}} |
| [[Category:Autoimmune diseases]]
| |
| [[Category:Gastroenterology]]
| |
| [[Category:Genetic disorders]]
| |
| [[Category:Malnutrition]]
| |
| [[Category:Pediatrics]]
| |
| [[Category:Mature chapter]]
| |
|
| |
| {{Link FA|de}} | | {{Link FA|de}} |
|
| |
|
| Line 367: |
Line 64: |
| {{WikiDoc Help Menu}} | | {{WikiDoc Help Menu}} |
| {{WikiDoc Sources}} | | {{WikiDoc Sources}} |
| | |
| | [[Category:Gastroenterology]] |
| | [[Category:Rheumatology]] |
| | [[Category:Autoimmune diseases]] |
| | [[Category:Genetic disorders]] |
| | [[Category:Malnutrition]] |
| | [[Category:Pediatrics]] |
| | [[Category:Dermatology]] |
| | [[Category:Up-To-Date]] |