Betrixaban: Difference between revisions
No edit summary |
No edit summary |
||
| Line 55: | Line 55: | ||
==Overview== | ==Overview== | ||
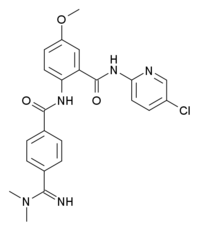
'''Betrixaban''' ([[International Nonproprietary Name|INN]], codenamed '''PRT-054,021''') is an [[anticoagulant]] drug which acts as a direct [[factor Xa]] inhibitor.<ref name="pmid19071881">{{cite journal |author=Eriksson BI, Quinlan DJ, Weitz JI |title=Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development |journal=Clinical Pharmacokinetics |volume=48 |issue=1 |pages=1–22 |year=2009 |pmid=19071881 |doi= |url=}}</ref> It is potent, orally active and highly selective for factor Xa, being selected from a group of similar compounds for its low [[hERG]] affinity.<ref name="pmid19297154">{{cite journal |author=Zhang P, Huang W, Wang L, Bao L, Jia ZJ, Bauer SM, Goldman EA, Probst GD, Song Y, Su T, Fan J, Wu Y, Li W, Woolfrey J, Sinha U, Wong PW, Edwards ST, Arfsten AE, Clizbe LA, Kanter J, Pandey A, Park G, Hutchaleelaha A, Lambing JL, Hollenbach SJ, Scarborough RM, Zhu BY |title=Discovery of betrixaban (PRT054021), N-(5-chloropyridin-2-yl)-2-(4-(N,N-dimethylcarbamimidoyl)benzamido)-5-methoxybenzamide, a highly potent, selective, and orally efficacious factor Xa inhibitor |journal=Bioorganic & Medicinal Chemistry Letters |volume=19 |issue=8 |pages=2179–85 |year=2009 |month=April |pmid=19297154 |doi=10.1016/j.bmcl.2009.02.111 |url=}}</ref> Betrixaban has undergone human [[clinical trials]] for prevention of embolism after knee surgery,<ref name="pmid19132191">{{cite journal |author=Turpie AG, Bauer KA, Davidson BL, Fisher WD, Gent M, Huo MH, Sinha U, Gretler DD |title=A randomized evaluation of betrixaban, an oral factor Xa inhibitor, for prevention of thromboembolic events after total knee replacement (EXPERT) |journal=Thrombosis and Haemostasis |volume=101 |issue=1 |pages=68–76 |year=2009 |month=January |pmid=19132191 |doi= |url=}}</ref> and prevention of [[stroke]] following [[atrial fibrillation]],<ref>{{cite pmid|20520539}}</ref> with promising results.<ref>{{cite pmid|19739042}}</ref> | '''Betrixaban''' ([[International Nonproprietary Name|INN]], codenamed '''PRT-054,021''') is an [[anticoagulant]] drug which acts as a direct [[factor Xa]] inhibitor.<ref name="pmid19071881">{{cite journal |author=Eriksson BI, Quinlan DJ, Weitz JI |title=Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development |journal=Clinical Pharmacokinetics |volume=48 |issue=1 |pages=1–22 |year=2009 |pmid=19071881 |doi= |url=}}</ref> It is potent, orally active and highly selective for factor Xa, being selected from a group of similar compounds for its low [[hERG]] affinity.<ref name="pmid19297154">{{cite journal |author=Zhang P, Huang W, Wang L, Bao L, Jia ZJ, Bauer SM, Goldman EA, Probst GD, Song Y, Su T, Fan J, Wu Y, Li W, Woolfrey J, Sinha U, Wong PW, Edwards ST, Arfsten AE, Clizbe LA, Kanter J, Pandey A, Park G, Hutchaleelaha A, Lambing JL, Hollenbach SJ, Scarborough RM, Zhu BY |title=Discovery of betrixaban (PRT054021), N-(5-chloropyridin-2-yl)-2-(4-(N,N-dimethylcarbamimidoyl)benzamido)-5-methoxybenzamide, a highly potent, selective, and orally efficacious factor Xa inhibitor |journal=Bioorganic & Medicinal Chemistry Letters |volume=19 |issue=8 |pages=2179–85 |year=2009 |month=April |pmid=19297154 |doi=10.1016/j.bmcl.2009.02.111 |url=}}</ref> Betrixaban has undergone human [[clinical trials]] for prevention of embolism after knee surgery,<ref name="pmid19132191">{{cite journal |author=Turpie AG, Bauer KA, Davidson BL, Fisher WD, Gent M, Huo MH, Sinha U, Gretler DD |title=A randomized evaluation of betrixaban, an oral factor Xa inhibitor, for prevention of thromboembolic events after total knee replacement (EXPERT) |journal=Thrombosis and Haemostasis |volume=101 |issue=1 |pages=68–76 |year=2009 |month=January |pmid=19132191 |doi= |url=}}</ref> and prevention of [[stroke]] following [[atrial fibrillation]],<ref>{{cite pmid|20520539}}</ref> with promising results.<ref>{{cite pmid|19739042}}</ref> | ||
==Chemical Properties== | |||
Betrixaban is a potent and specific inhibitor of human factor Xa (fXa) activity which acts by binding to the active site of fXa. Betrixaban as the maleate salt has a molecular weight (MW) of 567.98 | |||
==Pharmacokinetics== | |||
Betrixaban is available for oral administration as an immediate release capsule. It is rapidly absorbed with mean peak concentrations occurring 3-4 hours after oral administration. Oral bioavailability of an 80 mg dose is approximately 34% and protein binding is approximately 60%. The drug exhibits nonlinear kinetics within the expected therapeutic dosing range with greater than proportional increases in plasma concentrations occurring with increased dose; maximum plasma concentration (Cmax) and area under the curve (AUC) at steady-state increased approximately 3-fold when the dose was doubled from 40 mg to 80 mg. Excretion is mostly unchanged through the bile with renal clearance approximately 17% of the absorbed dose. When administered after a high-fat, high-calorie breakfast, Cmax and AUC were reduced by approximately 50% as compared to the fasting state. | |||
Betrixaban is not a substrate for major cytochrome P450 enzymes. It is a substrate for efflux proteins including permeability glycoprotein (P-glycoprotein or P-gp). When co-administered with the potent P-gp inhibitor, ketoconazole, betrixaban concentrations were increased approximately 2-fold. Based on population pharmacokinetic (PK) analysis from the Phase II EXPLORE Xa study, a similar effect was observed when betrixaban was given concomitantly with amiodarone. A drug interaction study with the P-gp substrate, digoxin, showed no significant interaction, and one with the inhibitor verapamil suggests that co-administration in fasted state increases both Cmax and exposure to betrixaban. | |||
==References== | ==References== | ||
Revision as of 00:10, 14 June 2012
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C23H22ClN5O3 |
| Molar mass | 451.905 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Betrixaban (INN, codenamed PRT-054,021) is an anticoagulant drug which acts as a direct factor Xa inhibitor.[1] It is potent, orally active and highly selective for factor Xa, being selected from a group of similar compounds for its low hERG affinity.[2] Betrixaban has undergone human clinical trials for prevention of embolism after knee surgery,[3] and prevention of stroke following atrial fibrillation,[4] with promising results.[5]
Chemical Properties
Betrixaban is a potent and specific inhibitor of human factor Xa (fXa) activity which acts by binding to the active site of fXa. Betrixaban as the maleate salt has a molecular weight (MW) of 567.98
Pharmacokinetics
Betrixaban is available for oral administration as an immediate release capsule. It is rapidly absorbed with mean peak concentrations occurring 3-4 hours after oral administration. Oral bioavailability of an 80 mg dose is approximately 34% and protein binding is approximately 60%. The drug exhibits nonlinear kinetics within the expected therapeutic dosing range with greater than proportional increases in plasma concentrations occurring with increased dose; maximum plasma concentration (Cmax) and area under the curve (AUC) at steady-state increased approximately 3-fold when the dose was doubled from 40 mg to 80 mg. Excretion is mostly unchanged through the bile with renal clearance approximately 17% of the absorbed dose. When administered after a high-fat, high-calorie breakfast, Cmax and AUC were reduced by approximately 50% as compared to the fasting state. Betrixaban is not a substrate for major cytochrome P450 enzymes. It is a substrate for efflux proteins including permeability glycoprotein (P-glycoprotein or P-gp). When co-administered with the potent P-gp inhibitor, ketoconazole, betrixaban concentrations were increased approximately 2-fold. Based on population pharmacokinetic (PK) analysis from the Phase II EXPLORE Xa study, a similar effect was observed when betrixaban was given concomitantly with amiodarone. A drug interaction study with the P-gp substrate, digoxin, showed no significant interaction, and one with the inhibitor verapamil suggests that co-administration in fasted state increases both Cmax and exposure to betrixaban.
References
- ↑ Eriksson BI, Quinlan DJ, Weitz JI (2009). "Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development". Clinical Pharmacokinetics. 48 (1): 1–22. PMID 19071881.
- ↑ Zhang P, Huang W, Wang L, Bao L, Jia ZJ, Bauer SM, Goldman EA, Probst GD, Song Y, Su T, Fan J, Wu Y, Li W, Woolfrey J, Sinha U, Wong PW, Edwards ST, Arfsten AE, Clizbe LA, Kanter J, Pandey A, Park G, Hutchaleelaha A, Lambing JL, Hollenbach SJ, Scarborough RM, Zhu BY (2009). "Discovery of betrixaban (PRT054021), N-(5-chloropyridin-2-yl)-2-(4-(N,N-dimethylcarbamimidoyl)benzamido)-5-methoxybenzamide, a highly potent, selective, and orally efficacious factor Xa inhibitor". Bioorganic & Medicinal Chemistry Letters. 19 (8): 2179–85. doi:10.1016/j.bmcl.2009.02.111. PMID 19297154. Unknown parameter
|month=ignored (help) - ↑ Turpie AG, Bauer KA, Davidson BL, Fisher WD, Gent M, Huo MH, Sinha U, Gretler DD (2009). "A randomized evaluation of betrixaban, an oral factor Xa inhibitor, for prevention of thromboembolic events after total knee replacement (EXPERT)". Thrombosis and Haemostasis. 101 (1): 68–76. PMID 19132191. Unknown parameter
|month=ignored (help) - ↑ PMID 20520539 (PMID 20520539)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 19739042 (PMID 19739042)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Pages with citations using unsupported parameters
- Pages with incomplete PMID references
- Drugs not assigned an ATC code
- Articles with changed CASNo identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Anticoagulants
- Drugs