Oxalate
Jump to navigation
Jump to search
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
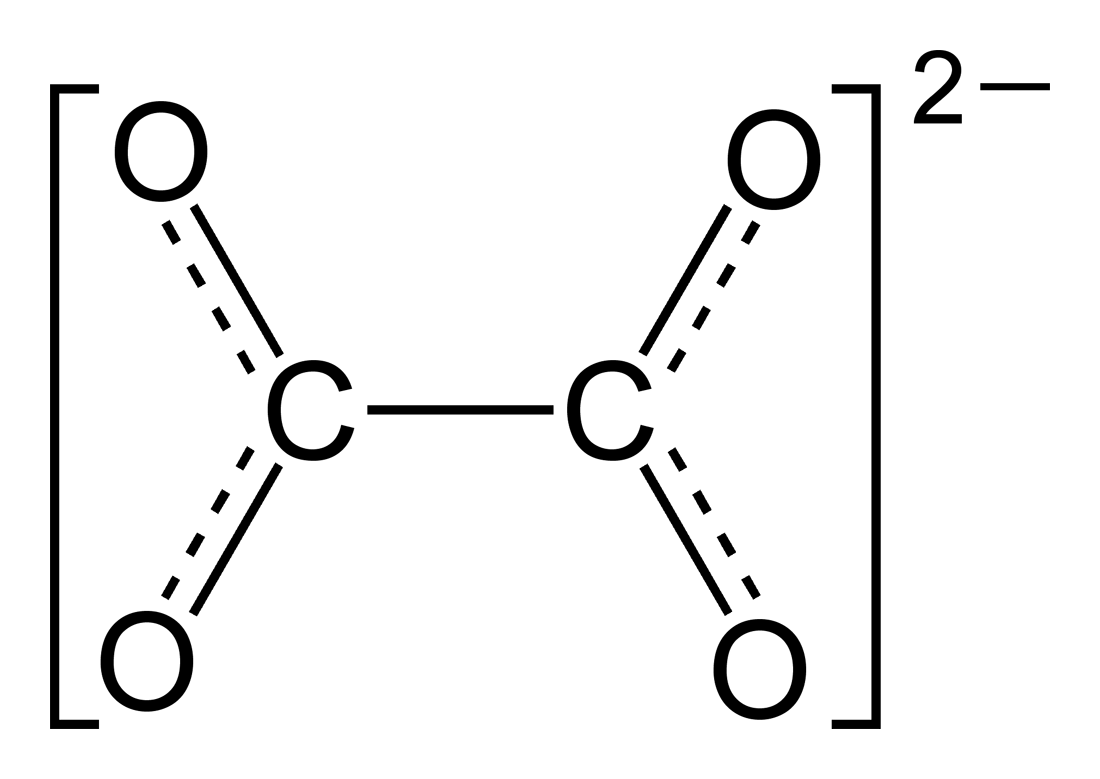
An oxalate (also ethanedioate) is a salt or ester of oxalic acid. As a salt, the oxalate anion has the chemical formula C2O42− or (COO)22−.
Consumption of oxalates (for example, the grazing of animals on oxalate-containing plants such as greasewood), or human consumption of Sorrel may result in kidney disease or even death due to oxalate poisoning.
The charge on oxalate allows it to act as a chelator of various positively charged metal ions.
Much of its other properties resemble oxalic acid.
Examples
- sodium oxalate - Na2C2O4
- calcium oxalate - CaC2O4, a major component of kidney stones
- dimethyl oxalate - (CH3)2C2O4
- phenyl oxalate ester - (C6H5)2C2O4
- potassium ferrioxalate - [K3[Fe(C2O4)3], an iron complex with oxalate ligands
- ammonium oxalate - (NH4)2C2O4
References