Aspiration pneumonia pathophysiology: Difference between revisions
m (Bot: Removing from Primary care) |
|||
| (30 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
{{CMG}}; {{AE}} {{SKA}}, {{SSH}} | {{CMG}}; {{AE}} {{SKA}}, {{SSH}} | ||
==Overview== | ==Overview== | ||
The mechanism behind damage of lung due to aspiration | Aspiration pneumonia is a common [[pneumonia]] among patients with risk factors including [[neurologic diseases]]. Microaspiration and macroaspiration of different materials are the primary cause of aspiration pneumonia. The mechanism behind damage of [[lung]] due to aspiration depends on the content of aspirate and the response of [[lung]] tissue to the content. Host factors including [[mucociliary clearance]], [[cough reflex]], and [[immune system]] might be impaired. [[Chemical pneumonitis]] usually occurs following aspiration of materials that are toxic to [[pulmonary]] tissue. There might be no [[Bacteria|bacterial]] or [[Virus|viral]] organisms involved. It is mostly associated with aspiration of [[gastric acid]]. In case of [[Pharynx|oropharyngeal]] secretions the damage is due to [[bacteria]] infecting and inducing [[inflammation]] in [[lung]] tissues. [[Foreign body]] aspiration might present acutely with mechanical [[obstruction]] or [[chemical pneumonitis]]. [[Lipid pneumonia|Lipoid pneumonia]] is caused by aspiration of mineral [[oil]] when used for [[constipation]] treatment. Following [[oil]] aspiration there is an [[Inflammation|inflammatory]] response with regional [[edema]], acute [[cough]], [[fever]], and [[dyspnea]]. Patients with [[Genetics|genetic]] syndromes and [[paralysis]] of lower [[cranial nerves]] might be prone to aspiration pneumonia. On [[gross pathology]], different aspirated particles might be seen. On microscopic histopathological analysis, aspirated material fragments, [[inflammation]], [[fibrosis]], and [[skeletal muscle]] fibers might be seen. | ||
==Pathophysiology== | == Pathophysiology == | ||
To understand the pathogenesis we have to review following physiological facts regarding aspiration pneumonia:<ref name=" | To understand the pathogenesis we have to review following [[Physiology|physiological]] facts regarding aspiration pneumonia:<ref name="pmid25732447">{{cite journal| author=Hu X, Lee JS, Pianosi PT, Ryu JH| title=Aspiration-related pulmonary syndromes. | journal=Chest | year= 2015 | volume= 147 | issue= 3 | pages= 815-823 | pmid=25732447 | doi=10.1378/chest.14-1049 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25732447 }}</ref><ref name="pmid198572242">{{cite journal| author=Japanese Respiratory Society| title=Aspiration pneumonia. | journal=Respirology | year= 2009 | volume= 14 Suppl 2 | issue= | pages= S59-64 | pmid=19857224 | doi=10.1111/j.1440-1843.2009.01578.x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19857224 }}</ref><ref name="pmid230520022">{{cite journal| author=Almirall J, Cabré M, Clavé P| title=Complications of oropharyngeal dysphagia: aspiration pneumonia. | journal=Nestle Nutr Inst Workshop Ser | year= 2012 | volume= 72 | issue= | pages= 67-76 | pmid=23052002 | doi=10.1159/000339989 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23052002 }}</ref><ref name="pmid9925081">{{cite journal| author=Marik PE, Careau P| title=The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. | journal=Chest | year= 1999 | volume= 115 | issue= 1 | pages= 178-83 | pmid=9925081 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9925081 }}</ref><ref name="pmid23598958">{{cite journal| author=Cordier JF, Cottin V| title=Neglected evidence in idiopathic pulmonary fibrosis: from history to earlier diagnosis. | journal=Eur Respir J | year= 2013 | volume= 42 | issue= 4 | pages= 916-23 | pmid=23598958 | doi=10.1183/09031936.00027913 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23598958 }}</ref><ref name="pmid29500665">{{cite journal| author=Shi X, Zheng J, Yan T| title=Computational redesign of human respiratory syncytial virus epitope as therapeutic peptide vaccines against pediatric pneumonia. | journal=J Mol Model | year= 2018 | volume= 24 | issue= 4 | pages= 79 | pmid=29500665 | doi=10.1007/s00894-018-3613-z | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=29500665 }}</ref><ref name="pmid28270104">{{cite journal| author=Shen CF, Wang SM, Ho TS, Liu CC| title=Clinical features of community acquired adenovirus pneumonia during the 2011 community outbreak in Southern Taiwan: role of host immune response. | journal=BMC Infect Dis | year= 2017 | volume= 17 | issue= 1 | pages= 196 | pmid=28270104 | doi=10.1186/s12879-017-2272-5 | pmc=5341368 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28270104 }}</ref><ref name="pmid21311332">{{cite journal| author=Marik PE| title=Pulmonary aspiration syndromes. | journal=Curr Opin Pulm Med | year= 2011 | volume= 17 | issue= 3 | pages= 148-54 | pmid=21311332 | doi=10.1097/MCP.0b013e32834397d6 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21311332 }}</ref><ref name="pmid257324472">{{cite journal| author=Hu X, Lee JS, Pianosi PT, Ryu JH| title=Aspiration-related pulmonary syndromes. | journal=Chest | year= 2015 | volume= 147 | issue= 3 | pages= 815-823 | pmid=25732447 | doi=10.1378/chest.14-1049 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25732447 }}</ref><ref name="DiBardinoWunderink2015">{{cite journal|last1=DiBardino|first1=David M.|last2=Wunderink|first2=Richard G.|title=Aspiration pneumonia: A review of modern trends|journal=Journal of Critical Care|volume=30|issue=1|year=2015|pages=40–48|issn=08839441|doi=10.1016/j.jcrc.2014.07.011}}</ref><ref name="TaylorFleming2013">{{cite journal|last1=Taylor|first1=Joanne K.|last2=Fleming|first2=Gillian B.|last3=Singanayagam|first3=Aran|last4=Hill|first4=Adam T.|last5=Chalmers|first5=James D.|title=Risk Factors for Aspiration in Community-acquired Pneumonia: Analysis of a Hospitalized UK Cohort|journal=The American Journal of Medicine|volume=126|issue=11|year=2013|pages=995–1001|issn=00029343|doi=10.1016/j.amjmed.2013.07.012}}</ref><ref name="HuLee2015">{{cite journal|last1=Hu|first1=Xiaowen|last2=Lee|first2=Joyce S.|last3=Pianosi|first3=Paolo T.|last4=Ryu|first4=Jay H.|title=Aspiration-Related Pulmonary Syndromes|journal=Chest|volume=147|issue=3|year=2015|pages=815–823|issn=00123692|doi=10.1378/chest.14-1049}}</ref><ref name="LanspaJones2013">{{cite journal|last1=Lanspa|first1=Michael J.|last2=Jones|first2=Barbara E.|last3=Brown|first3=Samuel M.|last4=Dean|first4=Nathan C.|title=Mortality, morbidity, and disease severity of patients with aspiration pneumonia|journal=Journal of Hospital Medicine|volume=8|issue=2|year=2013|pages=83–90|issn=15535592|doi=10.1002/jhm.1996}}</ref><ref name="LanspaJones20132">{{cite journal|last1=Lanspa|first1=Michael J.|last2=Jones|first2=Barbara E.|last3=Brown|first3=Samuel M.|last4=Dean|first4=Nathan C.|title=Mortality, morbidity, and disease severity of patients with aspiration pneumonia|journal=Journal of Hospital Medicine|volume=8|issue=2|year=2013|pages=83–90|issn=15535592|doi=10.1002/jhm.1996}}</ref><ref name="Marik20012">{{cite journal|last1=Marik|first1=Paul E.|title=Aspiration Pneumonitis and Aspiration Pneumonia|journal=New England Journal of Medicine|volume=344|issue=9|year=2001|pages=665–671|issn=0028-4793|doi=10.1056/NEJM200103013440908}}</ref><ref name="pmid19857224">{{cite journal| author=Japanese Respiratory Society| title=Aspiration pneumonia. | journal=Respirology | year= 2009 | volume= 14 Suppl 2 | issue= | pages= S59-64 | pmid=19857224 | doi=10.1111/j.1440-1843.2009.01578.x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19857224 }}</ref><ref name="pmid23052002">{{cite journal| author=Almirall J, Cabré M, Clavé P| title=Complications of oropharyngeal dysphagia: aspiration pneumonia. | journal=Nestle Nutr Inst Workshop Ser | year= 2012 | volume= 72 | issue= | pages= 67-76 | pmid=23052002 | doi=10.1159/000339989 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23052002 }}</ref> | ||
=== Mode of Transmission === | === Mode of Transmission === | ||
===== Inhalation of Aerosolized Droplets ===== | ===== Inhalation of Aerosolized Droplets ===== | ||
Inhalation of aerosolized droplets of 0.5 to 1 micrometer is the most common pathway of acquiring [[pneumonia]]. A few bacterial and viral infections are transmitted in this fashion. The lung can normally filter out particles between 0.5 to 2 micrometer by recruiting the | [[Inhalation]] of aerosolized droplets of 0.5 to 1 micrometer is the most common pathway of acquiring [[pneumonia]]. A few [[Bacteria|bacterial]] and [[Virus|viral]] infections are transmitted in this fashion. The lung can normally filter out particles between 0.5 to 2 micrometer by recruiting the [[Alveolus|alveolar]] [[macrophages]]. | ||
===== Microaspiration of Oropharyngeal Contents ===== | ===== Microaspiration of Oropharyngeal Contents ===== | ||
Aspiration of oropharyngeal contents containing pathogenic microorganisms is one of the mechanisms of acquiring [[pneumonia]]. It most commonly occurs in normal persons during sleep, in unconscious persons due to gastroesophageal reflux or impaired [[gag reflex]] and [[cough reflex]]. | [[Aspiration]] of [[Pharynx|oropharyngeal]] contents containing pathogenic [[Microorganism|microorganisms]] is one of the mechanisms of acquiring [[pneumonia]]. It most commonly occurs in normal persons during sleep, in unconscious persons due to [[Gastroesophageal reflux disease|gastroesophageal reflux]] or impaired [[gag reflex]] and [[cough reflex]]. | ||
=== Agent Specific Virulence Factors === | === Agent Specific Virulence Factors === | ||
Several strategies are evolved to evade host defense mechanisms and facilitate spreading before establishing an infection. | Several strategies are evolved to evade host defense mechanisms and facilitate spreading before establishing an [[infection]]. | ||
* [[Influenza virus]] possesses [[Neuraminidase|neuraminidases]] for cleavage of sialic acid residues on the cell surface and viral proteins, which prevent aggregation and facilitate propagation of viral particles. | * [[Influenza virus]] possesses [[Neuraminidase|neuraminidases]] for cleavage of [[sialic acid]] residues on the [[Cell surface molecule|cell surface]] and [[Virus|viral]] proteins, which prevent aggregation and facilitate propagation of [[Virus|viral]] particles. | ||
* ''[[Chlamydophila pneumoniae]]'' induces complete abortion of cilia motions which assists colonization at the [[respiratory epithelium]]. | * ''[[Chlamydophila pneumoniae]]'' induces complete abortion of [[Cilium|cilia]] motions which assists colonization at the [[respiratory epithelium]]. | ||
* ''[[Mycoplasma pneumoniae]]'' produces a virulence factor with [[ADP-ribosylation|ADP-ribosylating]] activity which is responsible for airway cellular damage and mucociliary dysfunction. | * ''[[Mycoplasma pneumoniae]]'' produces a virulence factor with [[ADP-ribosylation|ADP-ribosylating]] activity which is responsible for [[airway]] cellular damage and [[Mucociliary clearance|mucociliary]] dysfunction. | ||
* ''[[Haemophilus influenzae]]'', ''[[Streptococcus pneumoniae]]'', and ''[[Neisseria meningitidis]]'' produce [[Protease|proteases]] that split mucosal [[Immunoglobulin A|IgA]]. | * ''[[Haemophilus influenzae]]'', ''[[Streptococcus pneumoniae]]'', and ''[[Neisseria meningitidis]]'' produce [[Protease|proteases]] that split mucosal [[Immunoglobulin A|IgA]]. | ||
* ''[[Streptococcus pneumoniae]]'' possesses [[pneumolysin]] that aid the bacteria during colonization, by facilitating adherence to the host, during an invasion by damaging host cells, and during infection by interfering with the host immune response. | * ''[[Streptococcus pneumoniae]]'' possesses [[pneumolysin]] that aid the [[bacteria]] during colonization, by facilitating adherence to the host, during an invasion by damaging host cells, and during [[infection]] by interfering with the host [[Immune system|immune response]]. | ||
=== Host Factors === | === Host Factors === | ||
* The lungs can normally filter out large droplets of aerosols. | * The [[Lung|lungs]] can normally filter out large droplets of [[Aerosol|aerosols]]. | ||
* Smaller droplets of the size of 0.5 to 2 micrometer are deposited on the [[alveoli]] and then engulfed by alveolar macrophages. | * Smaller droplets of the size of 0.5 to 2 micrometer are deposited on the [[alveoli]] and then engulfed by [[Alveolus|alveolar]] [[Macrophage|macrophages]]. | ||
* These [[macrophages]] release [[cytokines]] and [[chemokines]], which also includes [[tumor necrosis factor-alpha]], [[interleukin]]-8 and [[Leukotriene|LTB4]]. | * These [[macrophages]] release [[cytokines]] and [[chemokines]], which also includes [[tumor necrosis factor-alpha]], [[interleukin]]-8 and [[Leukotriene|LTB4]]. | ||
* The [[neutrophils]] are recruited by these cells to eliminate these microorganisms. | * The [[neutrophils]] are recruited by these [[Cell (biology)|cells]] to eliminate these [[Microorganism|microorganisms]]. | ||
====== 1. Diminished Mucociliary Clearance ====== | ====== 1. Diminished Mucociliary Clearance ====== | ||
* The [[Respiratory epithelium#Ciliary Escalator|cilia]] lining the [[respiratory epithelium]] serve to move secreted [[mucus]] containing trapped foreign particles including pathogens towards the [[oropharynx]] for either expectoration or swallowing. | * The [[Respiratory epithelium#Ciliary Escalator|cilia]] lining the [[respiratory epithelium]] serve to move secreted [[mucus]] containing trapped foreign particles including [[Pathogen|pathogens]] towards the [[oropharynx]] for either expectoration or [[swallowing]]. | ||
* Elevated incidence of [[pneumonia]] in patients with genetic defects affecting [[mucociliary clearance]] such as [[primary ciliary dyskinesia]] suggests its role in the pathogenesis of community-acquired pneumonia. | * Elevated incidence of [[pneumonia]] in patients with [[Genetic disorder|genetic defects]] affecting [[mucociliary clearance]] such as [[primary ciliary dyskinesia]] suggests its role in the [[pathogenesis]] of [[community-acquired pneumonia]]. | ||
====== 2. Impaired Cough Reflex ====== | ====== 2. Impaired Cough Reflex ====== | ||
* [[Cough]], together with [[mucociliary clearance]], prevent pathogens from entering the lower [[respiratory tract]]. | * [[Cough]], together with [[mucociliary clearance]], prevent [[Pathogen|pathogens]] from entering the lower [[respiratory tract]]. | ||
* Cough suppression or [[cough reflex]] inhibition seen in patients with [[Cerebrovascular accident|cerebrovascular accidents]] and [[Overdose|drug overdosages]] is associated with an enhanced risk for [[aspiration pneumonia]]. | * [[Cough]] suppression or [[cough reflex]] inhibition seen in patients with [[Cerebrovascular accident|cerebrovascular accidents]] and [[Overdose|drug overdosages]] is associated with an enhanced risk for [[aspiration pneumonia]]. | ||
* Another relation to [[cough]] is [[Genetic polymorphism|genetic polymorphisms]] in the [[Angiotensin-converting enzyme|angiotensin-converting enzyme (ACE)]] gene. | * Another relation to [[cough]] is [[Genetic polymorphism|genetic polymorphisms]] in the [[Angiotensin-converting enzyme|angiotensin-converting enzyme (ACE)]] gene. | ||
* The role of [[cough]] in preventing [[pneumonia]] may be explained by a higher risk for developing [[pneumonia]] in [[Homozygote|homozygotes]] carrying [[Deletion|deletion/deletion (DD)]] [[genotype]] who are found to have lower levels of [[bradykinin]] and [[tachykinins]] such as [[substance P]]. | * The role of [[cough]] in preventing [[pneumonia]] may be explained by a higher risk for developing [[pneumonia]] in [[Homozygote|homozygotes]] carrying [[Deletion|deletion/deletion (DD)]] [[genotype]] who are found to have lower levels of [[bradykinin]] and [[tachykinins]] such as [[substance P]]. | ||
| Line 47: | Line 48: | ||
====== 3. Defective Immune System ====== | ====== 3. Defective Immune System ====== | ||
* [[Pathogen-associated molecular pattern|Pathogen-associated molecular patterns (PAMPs)]] are initially recognized by [[Toll-like receptor|Toll-like receptors (TLRs)]] and other [[Pattern recognition receptor|pattern-recognition receptors (PRRs)]] of the [[innate immune system]]. | * [[Pathogen-associated molecular pattern|Pathogen-associated molecular patterns (PAMPs)]] are initially recognized by [[Toll-like receptor|Toll-like receptors (TLRs)]] and other [[Pattern recognition receptor|pattern-recognition receptors (PRRs)]] of the [[innate immune system]]. | ||
* Effectors in the [[Acquired immunity|acquired immune system]] are involved in elimination of microorganisms and generation of immunological memory. | * Effectors in the [[Acquired immunity|acquired immune system]] are involved in elimination of [[Microorganism|microorganisms]] and generation of [[Immunology|immunological]] memory. | ||
* Other components in the immune system such as [[complement system]], [[Cytokine|cytokines]], and [[Collectin|collectins]], also mediate the defense against microorganisms causing pneumonia. | * Other components in the [[immune system]] such as [[complement system]], [[Cytokine|cytokines]], and [[Collectin|collectins]], also mediate the defense against [[Microorganism|microorganisms]] causing [[pneumonia]]. | ||
=== Chemical Pneumonitis === | |||
* [[Chemical pneumonitis]] usually occurs following aspiration of materials that are toxic to [[Lung|pulmonary]] tissue. There might be no [[Bacteria|bacterial]] or [[Virus|viral]] organisms involved. It is mostly associated with aspiration of [[gastric acid]]. | |||
* Following aspiration, there is onset of [[Respiratory failure|respiratory distress]] and [[cyanosis]] within 2 hours. | |||
* In animal and [[autopsy]] studies, following [[gastric acid]] aspiration, [[atelectasis]], peribronchial [[Bleeding|hemorrhage]], [[pulmonary edema]], and degeneration of bronchial [[Epithelium|epithelial cells]] initiates within three minutes. Release of [[Inflammation|proinflammatory]] [[Cytokine|cytokines]], especially [[tumor necrosis factor-alpha]] ([[Tumor necrosis factors|TNF]]) and [[Interleukin 8|interleukin-8]] causes [[Granulocyte|polymorphonuclear leukocytes]] and [[fibrin]] to fill the [[Alveolus|alveolar]] spaces after four hours. The [[lung]] become edematous and [[hemorrhagic]] with [[Alveolus|alveolar]] [[Consolidation (medicine)|consolidation]]. | |||
=== Bacterial Infection === | |||
* Another form of aspiration pneumonia is caused by less virulent oral [[bacteria]] that normally colonized in the upper [[Airway|airways]] or [[stomach]]. | |||
* [[Anaerobic respiration|Anaerobic]] bacteria are more involved in aspiration pneumonia. | |||
* ''[[Peptostreptococcus]]'', ''[[Fusobacterium|Fusobacterium nucleatum]]'', ''[[Prevotella]]'', ''[[Bacteroides]] melaninogenicus'', and other ''[[Bacteroides]] ''spp are the major [[Pathogen|pathogens]] that cause pulmonary [[Infection|infections]] following aspiration. However, the majority have mixed [[Aerobic organism|aerobe]] and [[Anaerobic organism|anaerobe]] infections. | |||
=== Foreign body aspiration === | |||
* [[Foreign body]] aspiration might present acutely with mechanical [[obstruction]] or [[chemical pneumonitis]]. | |||
* [[Foreign body]] aspiration is more common in children from one to three years of age. | |||
=== Lipoid Pneumonia === | |||
* [[Lipid pneumonia|Lipoid pneumonia]] is caused by aspiration of [[mineral oil]] when used for [[constipation]] treatment. | |||
* Patients usually have risk factors for aspiration. | |||
* Following [[oil]] aspiration there is an [[Inflammation|inflammatory]] response with regional edema and acute [[cough]], [[fever]], and [[dyspnea]]. | |||
* [[Fibrous connective tissue|Fibrous tissue]] encapsulates aspirated [[oils]] and develop intraalveolar hemorrhage. They will presents with a mass seen on imaging in an asymptomatic patient. | |||
==Genetics== | ==Genetics== | ||
*There are no genetic causes of aspiration pneumonia. | *There are no genetic causes of aspiration pneumonia. | ||
| Line 65: | Line 87: | ||
***[[Achalasia-addisonian syndrome|Triple-A syndrome]] | ***[[Achalasia-addisonian syndrome|Triple-A syndrome]] | ||
***Brown-Vialetto-Van Lare-syndrome | ***Brown-Vialetto-Van Lare-syndrome | ||
**Hereditary neuropathy | **Hereditary [[neuropathy]] | ||
==Gross Pathology== | ==Gross Pathology== | ||
*On gross pathology, [ | *On [[gross pathology]], different aspirated particles might be seen. | ||
{| | |||
| | |||
[[Image:Aspirated corn kernel (3791886968).jpg|300px|thumb|left|Aspirated corn kernel By Yale Rosen from USA - Uploaded by CFCF, CC BY-SA 2.0, Via Wikimedia<ref name="urlFile:Aspirated corn kernel (3791886968).jpg - Wikimedia Commons">{{cite web |url=https://commons.wikimedia.org/w/index.php?curid=31128322 |title=File:Aspirated corn kernel (3791886968).jpg - Wikimedia Commons |format= |work= |accessdate=}}</ref>]] | |||
| | |||
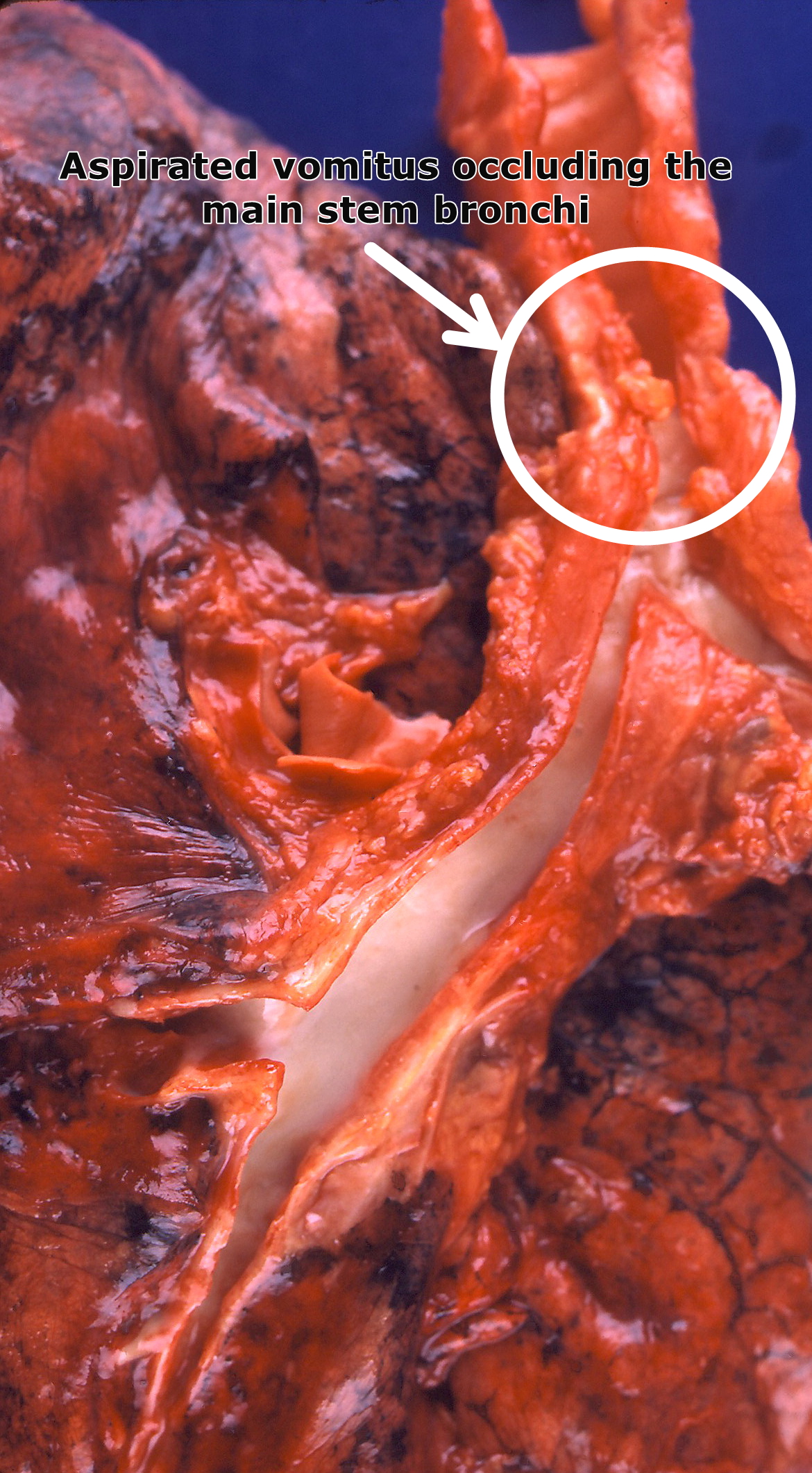
[[Image:Aspiration (4858360012).jpg|300px|thumb|left|Aspirated [[Nausea and vomiting|vomitus]] occluding the main stem [[Bronchus|bronchi]]. By Yale Rosen from USA - AspirationUploaded by CFCF, CC BY-SA 2.0, Via Wikimedia<ref name="urlFile:Aspiration (4858360012).jpg - Wikimedia Commons">{{cite web |url=https://commons.wikimedia.org/w/index.php?curid=31127980 |title=File:Aspiration (4858360012).jpg - Wikimedia Commons |format= |work= |accessdate=}}</ref>]] | |||
|} | |||
<br clear="left" /> | |||
==Microscopic Pathology== | ==Microscopic Pathology== | ||
*On microscopic histopathological analysis, [ | *On microscopic histopathological analysis, aspirated material fragments, [[inflammation]], [[fibrosis]], and [[skeletal muscle]] fibers might be seen. | ||
{{#ev:youtube|bTqgAfQv0p4}} | |||
{| | |||
| | |||
[[image:Aspiration pneumonia (5613726286).jpg|400px|left|thumb|Aspirated vegetable material surrounded by macrophages. This structure has a thick outer wall composed of cellulose surrounding a latticework of individual cells with thick cell walls composed of cellulose. By Yale Rosen from USA - Aspiration pneumoniaUploaded by CFCF, CC BY-SA 2.0, Via Wikimedia<ref name="urlFile:Aspiration pneumonia (5613726286).jpg - Wikimedia Commons">{{cite web |url=https://commons.wikimedia.org/w/index.php?curid=31127634 |title=File:Aspiration pneumonia (5613726286).jpg - Wikimedia Commons |format= |work= |accessdate=}}</ref>]] | |||
| | |||
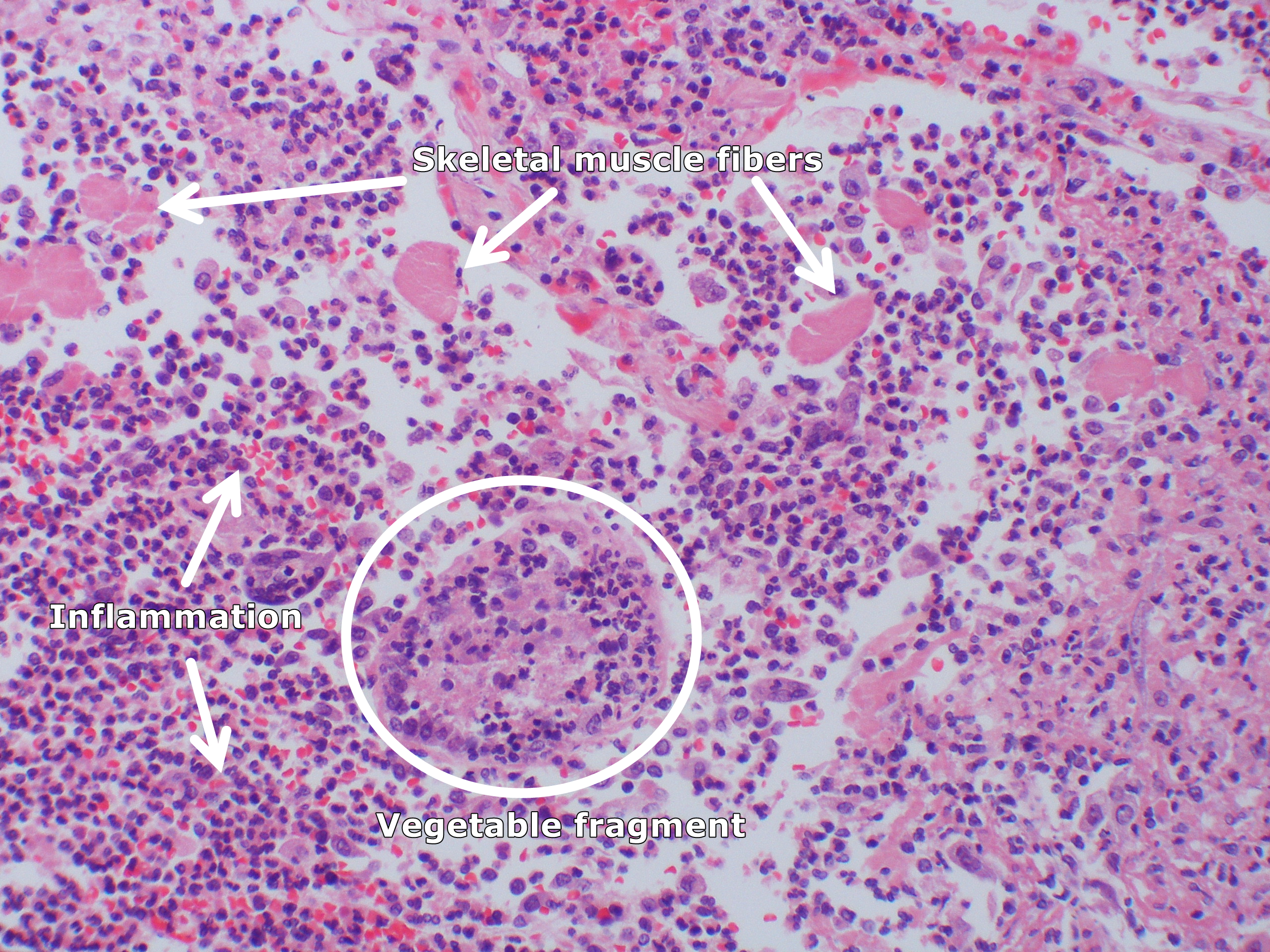
[[image:Aspiration pneumonia (5613146123).jpg|400px|left|thumb|Acute aspiration pneumonia with numemous [[Skeletal muscle]] fibers and a vegetable fragment infiltrated by polys. By Yale Rosen from USA - Aspiration pneumoniaUploaded by CFCF, CC BY-SA 2.0, Via Wikimedia<ref name="urlFile:Aspiration pneumonia (5613146123).jpg - Wikimedia Commons">{{cite web |url=https://commons.wikimedia.org/w/index.php?curid=31127636 |title=File:Aspiration pneumonia (5613146123).jpg - Wikimedia Commons |format= |work= |accessdate=}}</ref>]] | |||
|- | |||
| | |||
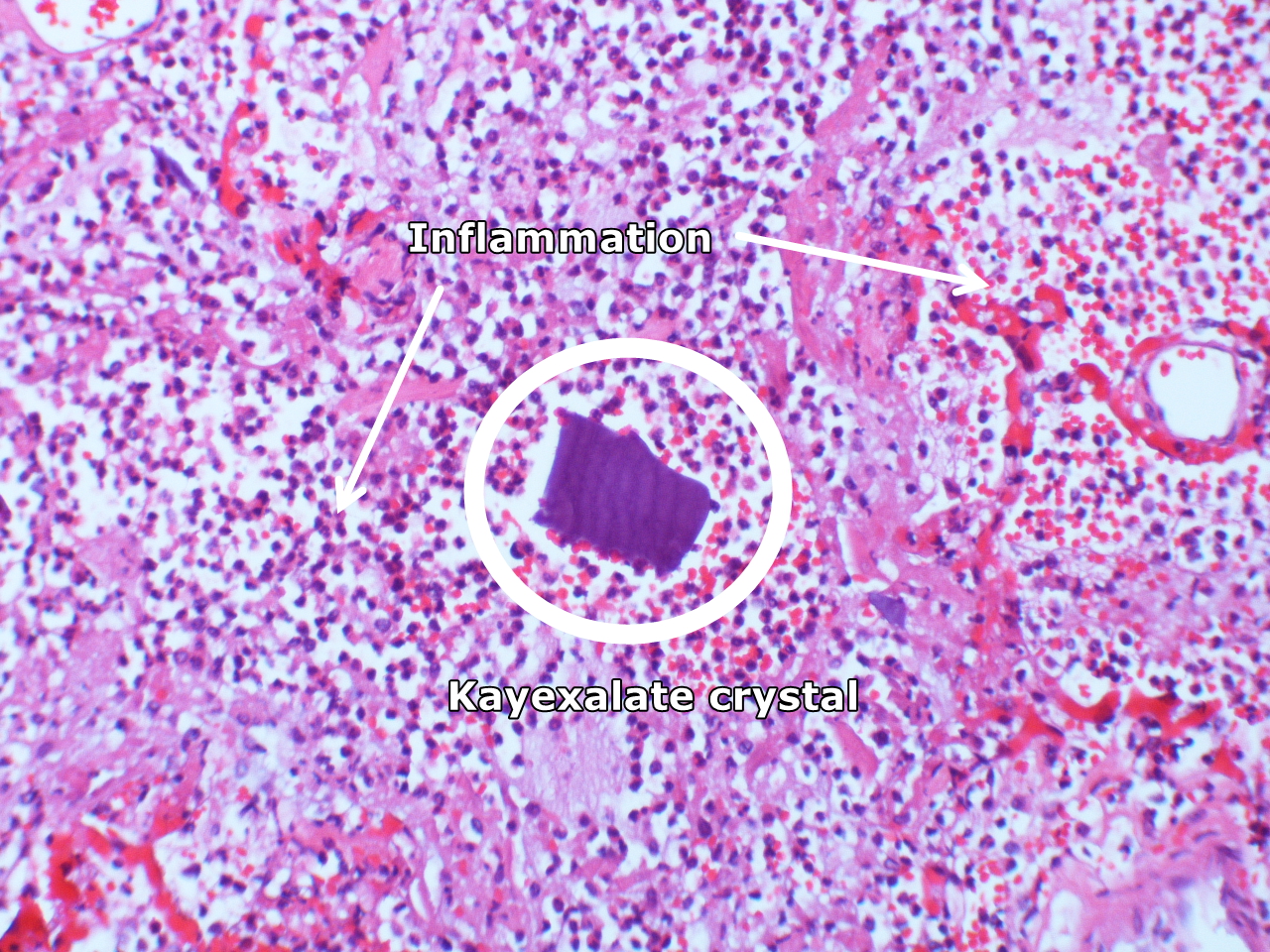
[[image:Kayexalate aspiration Case 125 (4692318776).jpg|400px|left|thumb|Intraalveolar kayexalate crystal; acute [[Pneumonitis]]. By Yale Rosen from USA - Kayexalate aspiration Case 125Uploaded by CFCF, CC BY-SA 2.0, Via Wikimedia<ref name="urlFile:Kayexalate aspiration Case 125 (4692318776).jpg - Wikimedia Commons">{{cite web |url=https://commons.wikimedia.org/w/index.php?curid=31128046 |title=File:Kayexalate aspiration Case 125 (4692318776).jpg - Wikimedia Commons |format= |work= |accessdate=}}</ref>]] | |||
| | |||
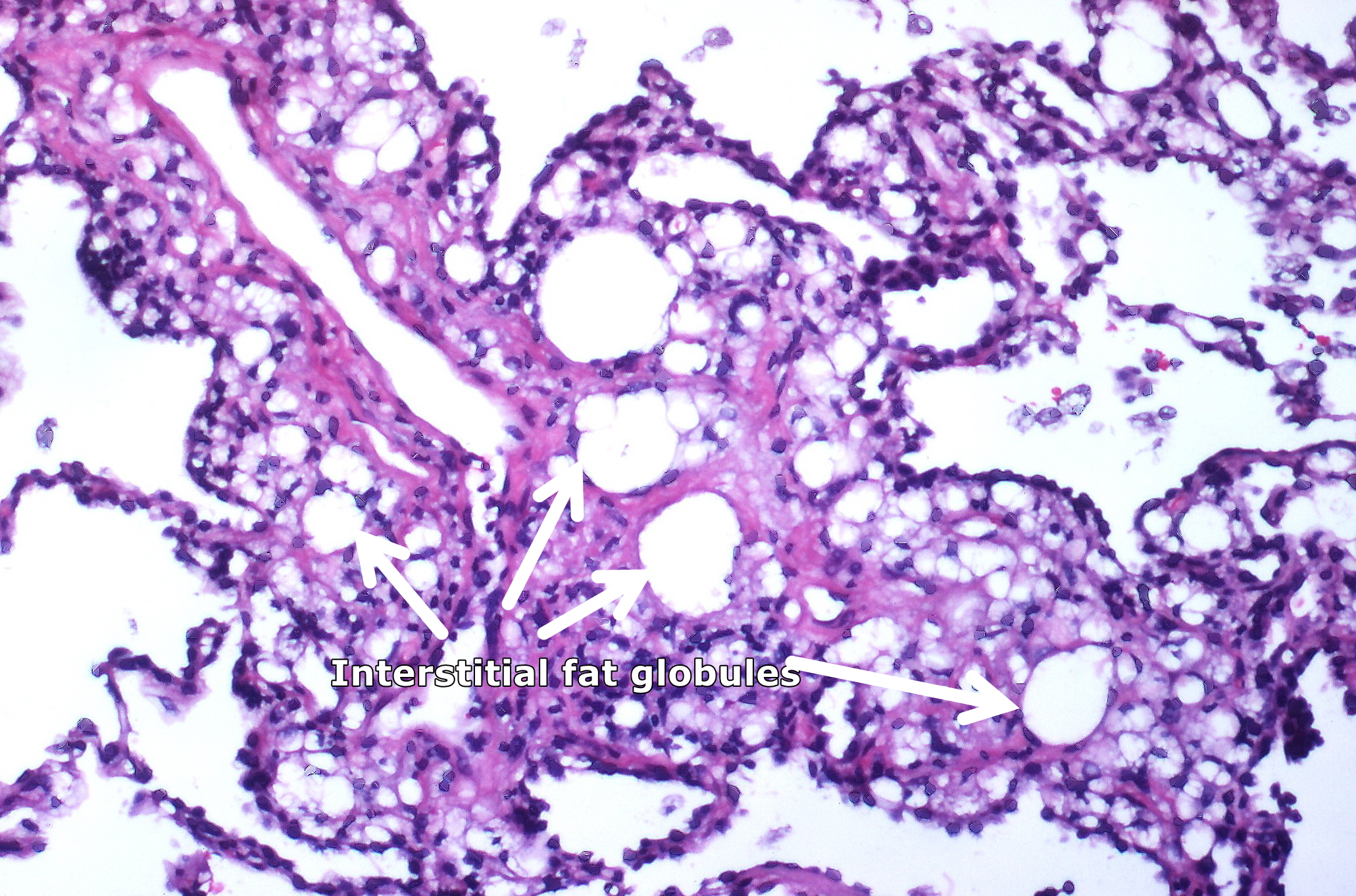
[[image:Lipid pneumonia, exogenous (3791887936).jpg|400px|left|thumb|Numerous interstitial fat globules of varying size accompanied by [[Inflammation]] and [[fibrosis]] is characteristic of chronic [[lipid pneumonia]] secondary to lipid aspiration. By Yale Rosen from USA - Lipid pneumonia, exogenousUploaded by CFCF, CC BY-SA 2.0, Via wikimedia<ref name="urlFile:Lipid pneumonia, exogenous (3791887936).jpg - Wikimedia Commons">{{cite web |url=https://commons.wikimedia.org/w/index.php?curid=31128316 |title=File:Lipid pneumonia, exogenous (3791887936).jpg - Wikimedia Commons |format= |work= |accessdate=}}</ref>]] | |||
|} | |||
<br clear="left" /> | |||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
| |||
[[Category:Medicine]] | |||
[[Category:Pulmonology]] | |||
[[Category: | [[Category:Up-To-Date]] | ||
[[Category:Emergency medicine]] | |||
Latest revision as of 20:29, 29 July 2020
|
Aspiration pneumonia Microchapters | |
|
Diagnosis | |
|---|---|
|
Treatment | |
|
Aspiration pneumonia pathophysiology On the Web | |
|
American Roentgen Ray Society Images of Aspiration pneumonia pathophysiology | |
|
Risk calculators and risk factors for Aspiration pneumonia pathophysiology | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sunny Kumar MD [2], Sadaf Sharfaei M.D.[3]
Overview
Aspiration pneumonia is a common pneumonia among patients with risk factors including neurologic diseases. Microaspiration and macroaspiration of different materials are the primary cause of aspiration pneumonia. The mechanism behind damage of lung due to aspiration depends on the content of aspirate and the response of lung tissue to the content. Host factors including mucociliary clearance, cough reflex, and immune system might be impaired. Chemical pneumonitis usually occurs following aspiration of materials that are toxic to pulmonary tissue. There might be no bacterial or viral organisms involved. It is mostly associated with aspiration of gastric acid. In case of oropharyngeal secretions the damage is due to bacteria infecting and inducing inflammation in lung tissues. Foreign body aspiration might present acutely with mechanical obstruction or chemical pneumonitis. Lipoid pneumonia is caused by aspiration of mineral oil when used for constipation treatment. Following oil aspiration there is an inflammatory response with regional edema, acute cough, fever, and dyspnea. Patients with genetic syndromes and paralysis of lower cranial nerves might be prone to aspiration pneumonia. On gross pathology, different aspirated particles might be seen. On microscopic histopathological analysis, aspirated material fragments, inflammation, fibrosis, and skeletal muscle fibers might be seen.
Pathophysiology
To understand the pathogenesis we have to review following physiological facts regarding aspiration pneumonia:[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17]
Mode of Transmission
Inhalation of Aerosolized Droplets
Inhalation of aerosolized droplets of 0.5 to 1 micrometer is the most common pathway of acquiring pneumonia. A few bacterial and viral infections are transmitted in this fashion. The lung can normally filter out particles between 0.5 to 2 micrometer by recruiting the alveolar macrophages.
Microaspiration of Oropharyngeal Contents
Aspiration of oropharyngeal contents containing pathogenic microorganisms is one of the mechanisms of acquiring pneumonia. It most commonly occurs in normal persons during sleep, in unconscious persons due to gastroesophageal reflux or impaired gag reflex and cough reflex.
Agent Specific Virulence Factors
Several strategies are evolved to evade host defense mechanisms and facilitate spreading before establishing an infection.
- Influenza virus possesses neuraminidases for cleavage of sialic acid residues on the cell surface and viral proteins, which prevent aggregation and facilitate propagation of viral particles.
- Chlamydophila pneumoniae induces complete abortion of cilia motions which assists colonization at the respiratory epithelium.
- Mycoplasma pneumoniae produces a virulence factor with ADP-ribosylating activity which is responsible for airway cellular damage and mucociliary dysfunction.
- Haemophilus influenzae, Streptococcus pneumoniae, and Neisseria meningitidis produce proteases that split mucosal IgA.
- Streptococcus pneumoniae possesses pneumolysin that aid the bacteria during colonization, by facilitating adherence to the host, during an invasion by damaging host cells, and during infection by interfering with the host immune response.
Host Factors
- The lungs can normally filter out large droplets of aerosols.
- Smaller droplets of the size of 0.5 to 2 micrometer are deposited on the alveoli and then engulfed by alveolar macrophages.
- These macrophages release cytokines and chemokines, which also includes tumor necrosis factor-alpha, interleukin-8 and LTB4.
- The neutrophils are recruited by these cells to eliminate these microorganisms.
1. Diminished Mucociliary Clearance
- The cilia lining the respiratory epithelium serve to move secreted mucus containing trapped foreign particles including pathogens towards the oropharynx for either expectoration or swallowing.
- Elevated incidence of pneumonia in patients with genetic defects affecting mucociliary clearance such as primary ciliary dyskinesia suggests its role in the pathogenesis of community-acquired pneumonia.
2. Impaired Cough Reflex
- Cough, together with mucociliary clearance, prevent pathogens from entering the lower respiratory tract.
- Cough suppression or cough reflex inhibition seen in patients with cerebrovascular accidents and drug overdosages is associated with an enhanced risk for aspiration pneumonia.
- Another relation to cough is genetic polymorphisms in the angiotensin-converting enzyme (ACE) gene.
- The role of cough in preventing pneumonia may be explained by a higher risk for developing pneumonia in homozygotes carrying deletion/deletion (DD) genotype who are found to have lower levels of bradykinin and tachykinins such as substance P.
3. Defective Immune System
- Pathogen-associated molecular patterns (PAMPs) are initially recognized by Toll-like receptors (TLRs) and other pattern-recognition receptors (PRRs) of the innate immune system.
- Effectors in the acquired immune system are involved in elimination of microorganisms and generation of immunological memory.
- Other components in the immune system such as complement system, cytokines, and collectins, also mediate the defense against microorganisms causing pneumonia.
Chemical Pneumonitis
- Chemical pneumonitis usually occurs following aspiration of materials that are toxic to pulmonary tissue. There might be no bacterial or viral organisms involved. It is mostly associated with aspiration of gastric acid.
- Following aspiration, there is onset of respiratory distress and cyanosis within 2 hours.
- In animal and autopsy studies, following gastric acid aspiration, atelectasis, peribronchial hemorrhage, pulmonary edema, and degeneration of bronchial epithelial cells initiates within three minutes. Release of proinflammatory cytokines, especially tumor necrosis factor-alpha (TNF) and interleukin-8 causes polymorphonuclear leukocytes and fibrin to fill the alveolar spaces after four hours. The lung become edematous and hemorrhagic with alveolar consolidation.
Bacterial Infection
- Another form of aspiration pneumonia is caused by less virulent oral bacteria that normally colonized in the upper airways or stomach.
- Anaerobic bacteria are more involved in aspiration pneumonia.
- Peptostreptococcus, Fusobacterium nucleatum, Prevotella, Bacteroides melaninogenicus, and other Bacteroides spp are the major pathogens that cause pulmonary infections following aspiration. However, the majority have mixed aerobe and anaerobe infections.
Foreign body aspiration
- Foreign body aspiration might present acutely with mechanical obstruction or chemical pneumonitis.
- Foreign body aspiration is more common in children from one to three years of age.
Lipoid Pneumonia
- Lipoid pneumonia is caused by aspiration of mineral oil when used for constipation treatment.
- Patients usually have risk factors for aspiration.
- Following oil aspiration there is an inflammatory response with regional edema and acute cough, fever, and dyspnea.
- Fibrous tissue encapsulates aspirated oils and develop intraalveolar hemorrhage. They will presents with a mass seen on imaging in an asymptomatic patient.
Genetics
- There are no genetic causes of aspiration pneumonia.
- However, patients with genetic syndromes and paralysis of following lower cranial nerves might be prone to aspiration pneumonia.[16][17]
- Cranial nerve 9 (glossopharyngeal)
- Cranial nerve 10 (vagal)
- Cranial nerve 11 (accessory)
- Cranial nerve 12 (hypoglossal)
- Associated genetic syndromes are as follow:
- Cerebral palsy
- Amyotrophic lateral sclerosis (ALS)
- Spinal muscular atrophy
- Bulbospinal muscular atrophy (BSMA)
- Unclassified motor neuron diseases such as:
- Sandhoff disease
- Triple-A syndrome
- Brown-Vialetto-Van Lare-syndrome
- Hereditary neuropathy
Gross Pathology
- On gross pathology, different aspirated particles might be seen.
 |
 |
Microscopic Pathology
- On microscopic histopathological analysis, aspirated material fragments, inflammation, fibrosis, and skeletal muscle fibers might be seen.
{{#ev:youtube|bTqgAfQv0p4}}
 |
 |
 |
 |
References
- ↑ Hu X, Lee JS, Pianosi PT, Ryu JH (2015). "Aspiration-related pulmonary syndromes". Chest. 147 (3): 815–823. doi:10.1378/chest.14-1049. PMID 25732447.
- ↑ Japanese Respiratory Society (2009). "Aspiration pneumonia". Respirology. 14 Suppl 2: S59–64. doi:10.1111/j.1440-1843.2009.01578.x. PMID 19857224.
- ↑ Almirall J, Cabré M, Clavé P (2012). "Complications of oropharyngeal dysphagia: aspiration pneumonia". Nestle Nutr Inst Workshop Ser. 72: 67–76. doi:10.1159/000339989. PMID 23052002.
- ↑ Marik PE, Careau P (1999). "The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study". Chest. 115 (1): 178–83. PMID 9925081.
- ↑ Cordier JF, Cottin V (2013). "Neglected evidence in idiopathic pulmonary fibrosis: from history to earlier diagnosis". Eur Respir J. 42 (4): 916–23. doi:10.1183/09031936.00027913. PMID 23598958.
- ↑ Shi X, Zheng J, Yan T (2018). "Computational redesign of human respiratory syncytial virus epitope as therapeutic peptide vaccines against pediatric pneumonia". J Mol Model. 24 (4): 79. doi:10.1007/s00894-018-3613-z. PMID 29500665.
- ↑ Shen CF, Wang SM, Ho TS, Liu CC (2017). "Clinical features of community acquired adenovirus pneumonia during the 2011 community outbreak in Southern Taiwan: role of host immune response". BMC Infect Dis. 17 (1): 196. doi:10.1186/s12879-017-2272-5. PMC 5341368. PMID 28270104.
- ↑ Marik PE (2011). "Pulmonary aspiration syndromes". Curr Opin Pulm Med. 17 (3): 148–54. doi:10.1097/MCP.0b013e32834397d6. PMID 21311332.
- ↑ Hu X, Lee JS, Pianosi PT, Ryu JH (2015). "Aspiration-related pulmonary syndromes". Chest. 147 (3): 815–823. doi:10.1378/chest.14-1049. PMID 25732447.
- ↑ DiBardino, David M.; Wunderink, Richard G. (2015). "Aspiration pneumonia: A review of modern trends". Journal of Critical Care. 30 (1): 40–48. doi:10.1016/j.jcrc.2014.07.011. ISSN 0883-9441.
- ↑ Taylor, Joanne K.; Fleming, Gillian B.; Singanayagam, Aran; Hill, Adam T.; Chalmers, James D. (2013). "Risk Factors for Aspiration in Community-acquired Pneumonia: Analysis of a Hospitalized UK Cohort". The American Journal of Medicine. 126 (11): 995–1001. doi:10.1016/j.amjmed.2013.07.012. ISSN 0002-9343.
- ↑ Hu, Xiaowen; Lee, Joyce S.; Pianosi, Paolo T.; Ryu, Jay H. (2015). "Aspiration-Related Pulmonary Syndromes". Chest. 147 (3): 815–823. doi:10.1378/chest.14-1049. ISSN 0012-3692.
- ↑ Lanspa, Michael J.; Jones, Barbara E.; Brown, Samuel M.; Dean, Nathan C. (2013). "Mortality, morbidity, and disease severity of patients with aspiration pneumonia". Journal of Hospital Medicine. 8 (2): 83–90. doi:10.1002/jhm.1996. ISSN 1553-5592.
- ↑ Lanspa, Michael J.; Jones, Barbara E.; Brown, Samuel M.; Dean, Nathan C. (2013). "Mortality, morbidity, and disease severity of patients with aspiration pneumonia". Journal of Hospital Medicine. 8 (2): 83–90. doi:10.1002/jhm.1996. ISSN 1553-5592.
- ↑ Marik, Paul E. (2001). "Aspiration Pneumonitis and Aspiration Pneumonia". New England Journal of Medicine. 344 (9): 665–671. doi:10.1056/NEJM200103013440908. ISSN 0028-4793.
- ↑ 16.0 16.1 Japanese Respiratory Society (2009). "Aspiration pneumonia". Respirology. 14 Suppl 2: S59–64. doi:10.1111/j.1440-1843.2009.01578.x. PMID 19857224.
- ↑ 17.0 17.1 Almirall J, Cabré M, Clavé P (2012). "Complications of oropharyngeal dysphagia: aspiration pneumonia". Nestle Nutr Inst Workshop Ser. 72: 67–76. doi:10.1159/000339989. PMID 23052002.
- ↑ "File:Aspirated corn kernel (3791886968).jpg - Wikimedia Commons".
- ↑ "File:Aspiration (4858360012).jpg - Wikimedia Commons".
- ↑ "File:Aspiration pneumonia (5613726286).jpg - Wikimedia Commons".
- ↑ "File:Aspiration pneumonia (5613146123).jpg - Wikimedia Commons".
- ↑ "File:Kayexalate aspiration Case 125 (4692318776).jpg - Wikimedia Commons".
- ↑ "File:Lipid pneumonia, exogenous (3791887936).jpg - Wikimedia Commons".