Alirocumab
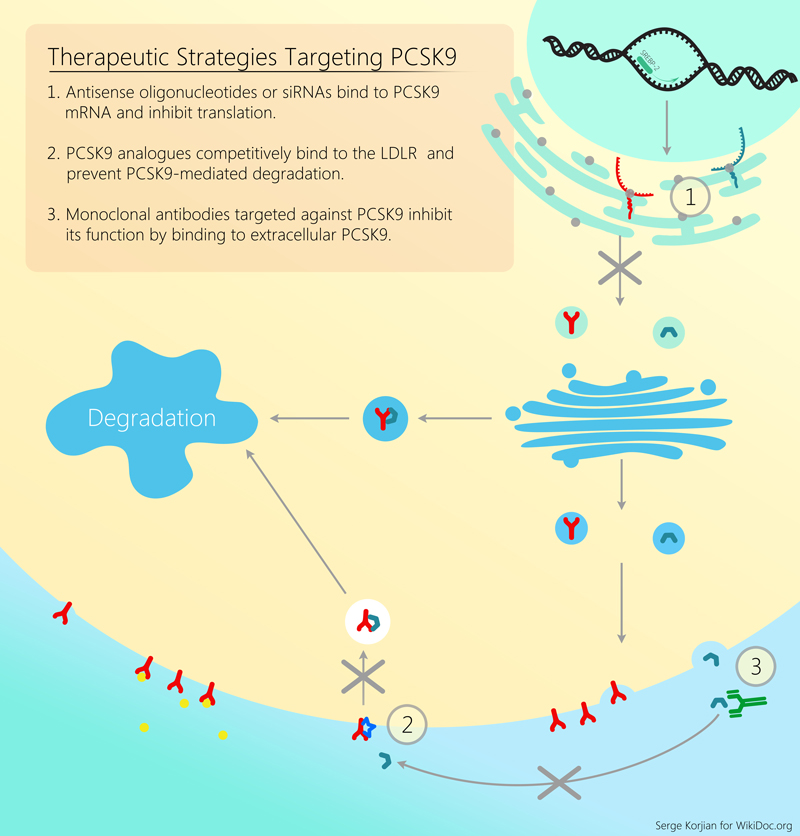
For a review of all PCSK9 inhibitors please click here
|
WikiDoc Resources for Alirocumab |
|
Articles |
|---|
|
Most recent articles on Alirocumab |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Alirocumab at Clinical Trials.gov Clinical Trials on Alirocumab at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Alirocumab
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Alirocumab Discussion groups on Alirocumab Patient Handouts on Alirocumab Directions to Hospitals Treating Alirocumab Risk calculators and risk factors for Alirocumab
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Alirocumab |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Alirocumab (REGN727 and SAR236553) is an investigational human monoclonal antibody that inhibits PCSK9 for the treatment of hypercholesterolemia.
Properties
Alirocumab is a human monoclonal antibody that binds proprotein convertase subtilisin/kexin type 9 (PCSK9). PCSK9 is a protein that attaches to surface LDL receptors and triggers their degradation. This reduces the ability of hepatocytes to uptake LDL cholesterol and subsequently leads to increased levels of circulating LDL. By binding PCSK9, alirocumab inhibits LDL receptor destruction and increases the endocytosis of LDL from the circulation. Pre-clinical studies, and early clinical trials have shown the efficacy and safety of alirocumab in decreasing LDL cholesterol as an add-on agent or as monotherapy.
Major Trials
Phase II Trials
Safety and efficacy in primary hypercholesterolemia with ongoing stable atorvastatin therapy
This randomized, double-blind, placebo-controlled phase 2 trial of 183 patients with LDL-C ≥100 mg/dL on stable-dose atorvastatin for 6 or more weeks randomized patients to a 12 week treatment course in either one of 6 arms (1:1:1:1:1:1): subcutaneous (SC) placebo every 2 weeks, 50 mg SC alirocumab every 2 weeks, 100 mg SC alirocumab every 2 weeks, 150 mg SC alirocumab every 2 weeks, 200 mg SC alirocumab every 4 weeks alternating with placebo, or 300 mg SC alirocumab every 4 weeks alternating with placebo. Alirocumab demonstrated a significant dose-related reduction in serum LDL-C (40%, 64%, and 72% with 50, 100, and 150 mg respectively, and 43% and 48% with 200 and 300 mg respectively compared with 5% in placebo) with no major safety signals. [2]
Safety and efficacy in heterozygous familial hypercholesterolaemia with ongoing stable-dose statin with or without ezetimibe therapy
This randomized, double-blind, placebo-controlled phase 2 trial of 77 adults with heterozygous familial hypercholesterolaemia and LDL-C concentrations of ≥100 mg/dL or higher on stable diet and statin dose, with or without ezetimibe therapy were randomized (1:1:1:1:1) to 5 different treatment arms for 12 weeks: 150 mg SC alirocumab every 4 weeks, 200 mg SC alirocumab every 4 weeks, 300 mg SC alirocumab every 4 weeks, 150 mg SC alirocumab every 2 weeks, or SC placebo. Randomization was stratified by baseline use of ezetimibe. Alirocumab demonstrated a significant reduction in LDL-C at week 12 (28.9%, 31.5%, and 42.5% for the 150, 200, and 300 mg every 4 weeks respectively, and 67.9% for the 150 mg every 2 weeks dose, compared with 10.7% in the placebo arm) with no significant safety signal. There were no increases >3 x ULN in hepatic transaminases or creatine kinase (CK).[3]
Phase III Trials
References
- ↑ Urban, D.; Pöss, J.; Böhm, M.; Laufs, U. (2013). "Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis". J Am Coll Cardiol. 62 (16): 1401–8. doi:10.1016/j.jacc.2013.07.056. PMID 23973703. Unknown parameter
|month=ignored (help) - ↑ McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA (2012). "Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy". J Am Coll Cardiol. 59 (25): 2344–53. doi:10.1016/j.jacc.2012.03.007. PMID 22463922.
- ↑ Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R; et al. (2012). "Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial". Lancet. 380 (9836): 29–36. doi:10.1016/S0140-6736(12)60771-5. PMID 22633824.