Alirocumab: Difference between revisions
Sergekorjian (talk | contribs) |
Sergekorjian (talk | contribs) |
||
| Line 28: | Line 28: | ||
ODYSSEY FHI and FH II were multicenter, double-blind, placebo-controlled trials that enrolled a total of 735 heterozygous familial hypercholesterolemia patients and randomized them to either SC alirocumab 75-150 mg every 2 weeks or matching placebo for a total of 78 weeks, on top of a background of lipid lowering therapy.The primary endpoint was the prrcent change in LDL-C from baseline to week 24. Alirocumab administration was associated with a 57.9% reduction compared to placebo in the FH I population (P<0.0001), and a 51.4% reduction compared to placebo in the FH II population (P<0.0001). Alirocumab was well tolerated and there were no safety concerns. | ODYSSEY FHI and FH II were multicenter, double-blind, placebo-controlled trials that enrolled a total of 735 heterozygous familial hypercholesterolemia patients and randomized them to either SC alirocumab 75-150 mg every 2 weeks or matching placebo for a total of 78 weeks, on top of a background of lipid lowering therapy.The primary endpoint was the prrcent change in LDL-C from baseline to week 24. Alirocumab administration was associated with a 57.9% reduction compared to placebo in the FH I population (P<0.0001), and a 51.4% reduction compared to placebo in the FH II population (P<0.0001). Alirocumab was well tolerated and there were no safety concerns. | ||
=====ODYSSEY - Long Term===== | =====ODYSSEY - Long Term===== | ||
Revision as of 15:43, 24 March 2015
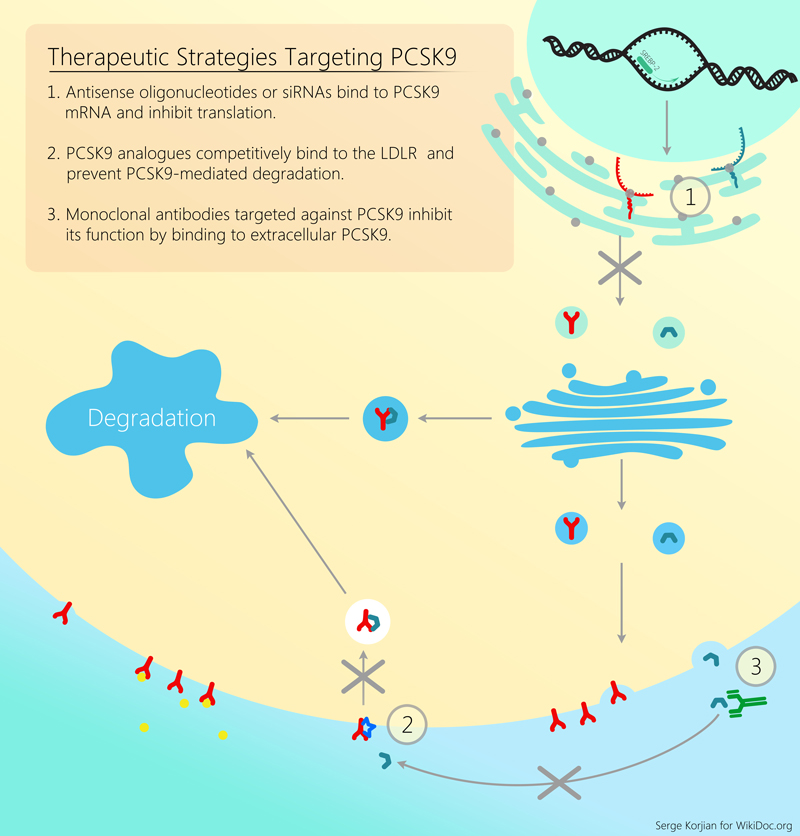
For a review of all PCSK9 inhibitors please click here
|
WikiDoc Resources for Alirocumab |
|
Articles |
|---|
|
Most recent articles on Alirocumab |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Alirocumab at Clinical Trials.gov Clinical Trials on Alirocumab at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Alirocumab
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Alirocumab Discussion groups on Alirocumab Patient Handouts on Alirocumab Directions to Hospitals Treating Alirocumab Risk calculators and risk factors for Alirocumab
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Alirocumab |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Alirocumab (REGN727 and SAR236553) is an investigational human monoclonal antibody that inhibits PCSK9 for the treatment of hypercholesterolemia.
Properties
Alirocumab is a human monoclonal antibody that binds proprotein convertase subtilisin/kexin type 9 (PCSK9). PCSK9 is a protein that attaches to surface LDL receptors and triggers their degradation. This reduces the ability of hepatocytes to uptake LDL cholesterol and subsequently leads to increased levels of circulating LDL. By binding PCSK9, alirocumab inhibits LDL receptor destruction and increases the endocytosis of LDL from the circulation. Pre-clinical studies, and early clinical trials have shown the efficacy and safety of alirocumab in decreasing LDL cholesterol as an add-on agent or as monotherapy.
Major Trials
Phase II Trials
Safety and efficacy of atorvastatin with or without alirocumab in primary hypercholesterolemia (NCT 01288469)
This randomized, double-blind, placebo-controlled phase 2 trial of 92 patients with LDL-C≥100 mg/dL after treatment with 10 mg of atorvastatin for at least 7 weeks randomized patients to 8 weeks of therapy with either 80 mg of atorvastatin daily plus 150 mg SC alirocumab once every 2 weeks, 10 mg of atorvastatin daily plus 150 mg alirocumab once every 2 weeks, or 80 mg of atorvastatin daily plus SC placebo once every 2 weeks. The trial demonstrated a significant 73.2% reduction from baseline serum LDL-C cholesterol with 80 mg of atorvastatin plus alirocumab compared with 17.3% with the 80 mg atorvastatin plus placebo. Ninety percent of the patients who received alirocumab reached LDL-C concentrations lower than 70 mg/dL compared with 17% of those receiving atorvastatin alone. There were no significant safety signals and the drug was well tolerated.[2]
Safety and efficacy in heterozygous familial hypercholesterolaemia with ongoing stable-dose statin with or without ezetimibe therapy (NCT 01266876)
This randomized, double-blind, placebo-controlled phase 2 trial of 77 adults with heterozygous familial hypercholesterolaemia and LDL-C concentrations of ≥100 mg/dL or higher on stable diet and statin dose, with or without ezetimibe therapy were randomized (1:1:1:1:1) to 5 different treatment arms for 12 weeks: 150 mg SC alirocumab every 4 weeks, 200 mg SC alirocumab every 4 weeks, 300 mg SC alirocumab every 4 weeks, 150 mg SC alirocumab every 2 weeks, or SC placebo. Randomization was stratified by baseline use of ezetimibe. Alirocumab demonstrated a significant reduction in LDL-C at week 12 (28.9%, 31.5%, and 42.5% for the 150, 200, and 300 mg every 4 weeks respectively, and 67.9% for the 150 mg every 2 weeks dose, compared with 10.7% in the placebo arm) with no significant safety signal. There were no increases >3 x ULN in hepatic transaminases or creatine kinase (CK).[3]
Safety and efficacy in primary hypercholesterolemia with ongoing stable atorvastatin therapy (NCT 01288443)
This randomized, double-blind, placebo-controlled phase 2 trial of 183 patients with LDL-C ≥100 mg/dL on stable-dose atorvastatin for 6 or more weeks randomized patients to a 12 week treatment course in either one of 6 arms (1:1:1:1:1:1): subcutaneous (SC) placebo every 2 weeks, 50 mg SC alirocumab every 2 weeks, 100 mg SC alirocumab every 2 weeks, 150 mg SC alirocumab every 2 weeks, 200 mg SC alirocumab every 4 weeks alternating with placebo, or 300 mg SC alirocumab every 4 weeks alternating with placebo. Alirocumab demonstrated a significant dose-related reduction in serum LDL-C (40%, 64%, and 72% with 50, 100, and 150 mg respectively, and 43% and 48% with 200 and 300 mg respectively compared with 5% in placebo) with no major safety signals. [4]
Phase III Trials
ODYSSEY - COMBO II
COMBO II was a randomized, double-blind, double-dummy, trial that randomized 720 patients with elevated CV risk and LDL-C despite maximal statins use to either SC alirocumab 75 mg every 2 weeks (and PO placebo) or PO ezetimibe 10 mg daily (and SC placebo). Alirocumab was generally well tolerated, with no reported safety signals. Alirocumab treatment was associated with a significantly higher reduction in mean LDL-C values from baselines at week 24 (50.6 ± 1.4% for alirocumab vs. 20.7 ± 1.9% for ezetimibe; P < 0.0001). Patients on alirocumab were more likely to achieve LDL-C <1.8 mmol/L (77.0% vs. 45.6%; P < 0.0001). At week 24, mean LDL-C levels were 1.3 ± 0.04 mmol/L among patients receiving alirocumab, and 2.1 ± 0.05 mmol/L among patients receiving ezetimibe.[5]
ODYSSEY - FH I & FH II
ODYSSEY FHI and FH II were multicenter, double-blind, placebo-controlled trials that enrolled a total of 735 heterozygous familial hypercholesterolemia patients and randomized them to either SC alirocumab 75-150 mg every 2 weeks or matching placebo for a total of 78 weeks, on top of a background of lipid lowering therapy.The primary endpoint was the prrcent change in LDL-C from baseline to week 24. Alirocumab administration was associated with a 57.9% reduction compared to placebo in the FH I population (P<0.0001), and a 51.4% reduction compared to placebo in the FH II population (P<0.0001). Alirocumab was well tolerated and there were no safety concerns.
ODYSSEY - Long Term
References
- ↑ Urban, D.; Pöss, J.; Böhm, M.; Laufs, U. (2013). "Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis". J Am Coll Cardiol. 62 (16): 1401–8. doi:10.1016/j.jacc.2013.07.056. PMID 23973703. Unknown parameter
|month=ignored (help) - ↑ Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA (2012). "Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia". N Engl J Med. 367 (20): 1891–900. doi:10.1056/NEJMoa1201832. PMID 23113833.
- ↑ Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R; et al. (2012). "Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial". Lancet. 380 (9836): 29–36. doi:10.1016/S0140-6736(12)60771-5. PMID 22633824.
- ↑ McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA (2012). "Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy". J Am Coll Cardiol. 59 (25): 2344–53. doi:10.1016/j.jacc.2012.03.007. PMID 22463922.
- ↑ Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R; et al. (2015). "Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial". Eur Heart J. doi:10.1093/eurheartj/ehv028. PMID 25687353.