Primary peritoneal cancer
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Synonyms and keywords: Primary peritoneal neoplasm; Primary peritoneal serous carcinoma; Primary peritoneal malignancy; Primary peritoneal tumors; Serous surface papillary carcinoma; Primary peritoneal carcinoma; Extra-ovarian serous carcinoma; Primary serous papillary carcinoma; Psammomacarcinoma
Overview
Primary peritoneal cancer is a cancer of the cells lining the peritoneum or abdominal cavity. It is indistinguishable histologically from primary epithelial ovarian carcinoma: serous type. Precise causes are unknown, link with certain variants of BRCA 1 and BRCA 2 mutation has been described. Primary peritoneal cancer commonly affects females more than males, older than 30 years of age (mean age of diagnosis is 67 years old) and Caucasian Americans are noted to be more affected than African Americans. Diagnostics involves immunohistochemistry, imaging and tissue biopsy. Surgical debulking is the primary treatment for extra-ovarian primary peritoneal carcinoma. Staging is usually done intraoperatively. Chemotherapy is done after optimal tumor debulking.
Pathophysiology
- Primary peritoneal cancer is a cancer of the cells lining the peritoneum or abdominal cavity.[1]
- They are histologically indistinguishable from primary serous ovarian carcinoma.
- Some studies indicate that between 7 to 20 percent of initially diagnosed epithelial ovarian cancers could be properly reclassified as primary peritoneal cancers.
- Primary serous ovarian carcinoma and primary peritoneal carcinoma share the same molecular alterations. This involves mutations in the TP53 gene which produces abnormal P53 proteins.
Classification
- Primary peritoneal neoplasms comprise of an uncommon group of heterogeneous entities that include:
- Mesothelial derivatives:
- Primary (malignant) peritoneal mesothelioma
- Primary peritoneal multicystic mesothelioma
- Primary peritoneal well differentiated papillary mesothelioma
- Primary peritoneal adenomatoid tumor
- Epithelial derivatives:
- Primary peritoneal serous carcinoma / primary peritoneal papillary serous carcinoma
- Primary peritoneal serous borderline tumor
- Smooth muscle cell derivatives
- Diffuse peritoneal leiomyomatosis (leiomyomatosis peritonialis disseminata)
- Others
- Desmoplastic small round cell tumor arising from the peritoneum
- Solitary fibrous tumor arising the abdomen/peritoneum
- Peritoneal lymphangioma
- Mesothelial derivatives:
Embryological Basis of Pathogenesis
- Ovarian and peritoneal epithelium share common embryonal origin, which is the coelomic epithelium. Coelomic epithelium is thought to be of mesonephric origin. With the overall point being that normal ovarian and peritoneal tissue is derived from the mesonephros. On the contrary, fallopian tube epithelium, endometrium, and endocervix are related to paramesonephros (Müllerian duct). Surprisingly, epithelial ovarian cancer and primary peritoneal cancer are histologically similar to the mullerian epithelium; not their embryonal origin, the mesonephros. This observation suggests that either a metaplasia has occurred or Mullerian remnants have been left behind in coelomic epithelium, which has turned oncogenic.
- Newer studies have identified WT1 as a marker differentiating uterine papillary serous carcinoma from the uterus to primary peritoneal carcinoma and papillary serous carcinoma of the ovaries.
Genetics
- Although the precise cause is unknown, a link with certain variants of BRCA1 and BRCA 2 mutation has been described.[2] Furthermore, women with BRCA1 and BRCA2 mutation have a 5% risk of developing primary peritoneal cancer even after prophylactic oophorectomy.
- Primary peritoneal carcinoma shows similar rates of tumor suppressor gene dysfunction as ovarian cancer (including P53, BRCA and WT1) and can also show an increased expression of HER-2/neu.
- An association with vascular endothelial growth factor has been observed.[3]
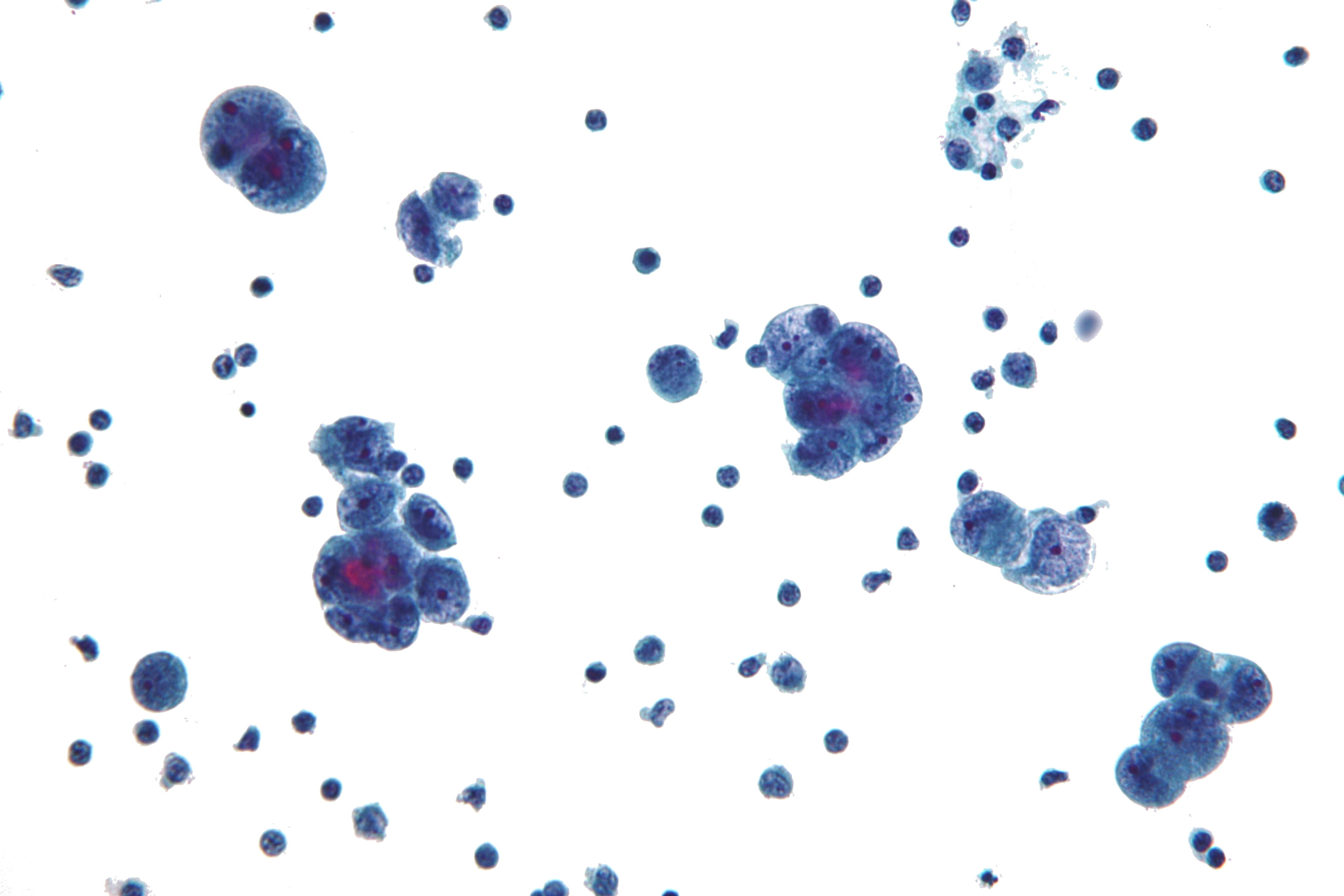
Microscopic Pathology
Gross Pathology
- Peritoneum shows diffuse thickening with irregular surface studded with multiple nodules over the surface. Serial sectioning shows grey-white to grey-yellow uniformly solid firm to hard peritoneal flap with variable thickness. [3]
Causes
- The cause of primary peritoneal cancer has not been identified.
Differentiating Primary peritoneal cancer from other Diseases
- Primary peritoneal cancer must be differentiated from:
- Asbestos
- Fibroid
- Pregnancy
- Pelvic inflammatory disease
- Primary serous ovarian carcinoma
- Ovarian cyst
Demographics
Age
- Primary peritoneal cancer commonly affects individuals older than 30 years of age.
Gender
- Females are more commonly affected with primary peritoneal cancer than males.
Race
- Caucasian Americans affected more than African Americans.
Natural History, Complications and Prognosis
- Primary peritoneal cancer is associated with a particularly poor prognosis. Median survival period is 12-25 months.
- Primary peritoneal cancer associated with BRCA mutations is demonstrated to have improved survival this is postulated to be because of improved survival of BRCA2 mutation carrier.
- Patients with BRCA-associated primary peritoneal cancer was shown to have a personal history of breast cancer as compared to sporadic cases of primary peritoneal cancer.
Diagnosis
Symptoms
- Ascitis
- Weight loss
- Abdominal mass
- Abdominal pain
- Indigestion
- Nausea
- Shortness of breath
Physical Examination
Appearance of the Patient
- Patients with primary peritoneal cancer usually appear cachectic.
Abdomen
- The presence of a large ill defined anterior abdominal mass on physical examination is suggestive of primary peritoneal cancer.
Lab Findings
Elevated albumin levels have been detected among patients with primary peritoneal cancer.[5]
Immunohistochemistry[6]
- Positive immunostaining for the following genetic markers:
- Negative for:
Imaging
- Computed Tomography (CT) scan and MRI findings in primary peritoneal cancer will show:
- Ascites
- Peritoneal thickening and enhancement
- Peritoneal nodules or bulky mass lesions
- Lymph node involvement
- Distant metastases
- Especially to liver
Biopsy
- Percutaneous ultrasound guided omental biospy is done on selected cases.
- Seeding of tumor cells along the biopsy track is a theoretical concern but no such case have been reported to date.
- No significant complications have been recorded.
- CT-scan/MRI are imaging preferred to be done prior to biopsy to delineate the extent of the tumor.
Treatment
- Prognosis and treatment is the same as for epithelial ovarian cancers.[7][8]
- Optimal tumor debulking followed by chemotherapy is the mode of treatment of primary peritoneal cancer.
Pharmacotherapy
- Preferred regimen: Paclitaxel 135 mg/m2 IV over 24 h on day 1 AND cisplatin 100 mg/m2 IP on day 2 AND paclitaxel 60 mg/m 2 IP on day 8 for 21 days for 6 cycles.[9](1)
- Preferred regimen (2): (Paclitaxel 135-175 mg/m2 IV infused over 3 hours AND carboplatin AUC 5-7.5 IV infused over 30-60 min every 21 days for three to six cycles) OR (Docetaxel 60-75 mg/m 2 IV infused over 1 hour) AND carboplatin AUC 5-6 IV infused over 1 hour every 21 days for three to six cycles[10][11][12]
Treatment of Recurrent Disease
Platinum-sensitive disease
- Preferred regimen(1): Paclitaxel 135-175 mg/m2 IV infused over 3 hours AND carboplatin AUC 5-6 IV infused over 1 hour every 21 days for six cycles[13]
- Preferred regimen(2): Docetaxel 60-75 mg/m2 IV infused over 1 hour AND carboplatin AUC 5 IV infused over 1 hour every 21 days for three to six cycles
- Preferred regimen(3): Pegylated liposomal doxorubicin 30 mg/m2 IV infused over 30 min AND carboplatin AUC 5 IV every 21 days for six cycles
- Preferred regimen(4): Gemcitabine 1000 mg/m2 IV on days 1 and 8 AND carboplatin AUC 4 on day 1 every 21 days for six cycles
- Note: Bevacizumab (15 mg/kg every 3 wk) may be added to the regimen
- Preferred regimen(5): Carboplatin AUC 2 IV push with paclitaxel 80 mg/m2 IV infused over 3 hours on days 1, 8, and 15
Platinum-resistant disease
- Preferred regimen(1): Pegylated liposomal doxorubicin 50 mg/m2 IV infused over 30 min every 21 days
- Preferred regimen(2): Topotecan 1.25 mg/m2 IV infused over 30 min on days 1-5 every 21 days
- Preferred regimen(3): Gemcitabine 1000 mg/m2 IV infused over 30 min on days 1 and 8 every 21 days
Combination Regimen
- Bevacizumab 10 mg/kg IV every 14 days in combination with one of the following IV chemotherapy regimens: paclitaxel, pegylated liposomal doxorubicin, or topotecan (topotecan is given weekly)
- Bevacizumab 15 mg/kg IV every 21 days in combination with topotecan (every 21 days)
Surgery
- Surgery is the primary treatment for extra-ovarian primary peritoneal carcinoma. The type of surgery done is surgical debulking, with the aim to remove as much tumor before doing chemotherapy. Staging is done at the same time as the surgery. Staging is the same with ovarian cancer.[14]
References
- ↑ Primary peritoneal cancer. Wikipedia (2015). https://en.wikipedia.org/wiki/Primary_peritoneal_carcinoma Accessed on January 5, 2016
- ↑ "Gynecologic Cancer Treatment — Primary Peritoneal Cancer — Dana-Farber Cancer Institute".
- ↑ Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI (November 2007). "Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study". J. Clin. Oncol. 25 (33): 5165–71. doi:10.1200/JCO.2007.11.5345. PMID 18024863.
- ↑ Image courtesy of wikipedia. Radiopaedia (original file ‘’here’’.Creative Commons BY-SA-NC
- ↑ Alphs HH, Zahurak ML, Bristow RE, Díaz-Montes TP (December 2006). "Predictors of surgical outcome and survival among elderly women diagnosed with ovarian and primary peritoneal cancer". Gynecol. Oncol. 103 (3): 1048–53. doi:10.1016/j.ygyno.2006.06.019. PMID 16876237.
- ↑ "Primary Peritoneal Serous Carcinoma: A Rare Case and Palliative Approach".
- ↑ "New Drug Combination for Ovarian and Primary Peritoneal Cancers - National Cancer Institute".
- ↑ "eMedicine — Peritoneal Cancer : Article by Wissam Bleibel".
- ↑ Foote EA, Postier RG, Greenfield RA, Bronze MS (2005). "Infectious Aortitis". Curr Treat Options Cardiovasc Med. 7 (2): 89–97. PMID 15935117.
- ↑ Walker, Joan L. (2009). "Intraperitoneal chemotherapy for ovarian cancer: 2009 goals". Gynecologic Oncology. 112 (3): 439–440. doi:10.1016/j.ygyno.2009.01.007. ISSN 0090-8258.
- ↑ Konner, J. A.; Grabon, D. M.; Gerst, S. R.; Iasonos, A.; Thaler, H.; Pezzulli, S. D.; Sabbatini, P. J.; Bell-McGuinn, K. M.; Tew, W. P.; Hensley, M. L.; Spriggs, D. R.; Aghajanian, C. A. (2011). "Phase II Study of Intraperitoneal Paclitaxel Plus Cisplatin and Intravenous Paclitaxel Plus Bevacizumab As Adjuvant Treatment of Optimal Stage II/III Epithelial Ovarian Cancer". Journal of Clinical Oncology. 29 (35): 4662–4668. doi:10.1200/JCO.2011.36.1352. ISSN 0732-183X.
- ↑ Ozols, R. F. (2003). "Phase III Trial of Carboplatin and Paclitaxel Compared With Cisplatin and Paclitaxel in Patients With Optimally Resected Stage III Ovarian Cancer: A Gynecologic Oncology Group Study". Journal of Clinical Oncology. 21 (17): 3194–3200. doi:10.1200/JCO.2003.02.153. ISSN 0732-183X.
- ↑ Armstrong, Deborah K.; Bundy, Brian; Wenzel, Lari; Huang, Helen Q.; Baergen, Rebecca; Lele, Shashikant; Copeland, Larry J.; Walker, Joan L.; Burger, Robert A. (2006). "Intraperitoneal Cisplatin and Paclitaxel in Ovarian Cancer". New England Journal of Medicine. 354 (1): 34–43. doi:10.1056/NEJMoa052985. ISSN 0028-4793.
- ↑ Primary peritoneal cancer. Canadian cancer society (2016). http://www.cancer.ca/en/cancer-information/cancer-type/ovarian/treatment/extra-ovarian-primary-peritoneal-carcinoma/?region=on Accessed on January 06, 2016