Botulinum antitoxin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Botulinum antitoxin is an human immunoglobulin that is FDA approved for the treatment of of infant botulism caused by toxin type A or B in patients below one year of age. Common adverse reactions include skin rash, chills, muscle cramps, back pain, fever, nausea, vomiting, and wheezing.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Botulinum antitoxin FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Botulinum antitoxin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Botulinum antitoxin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Infant botulism
- Botulism Immune Globulin®, Botulism Immune Globulin Intravenous (Human), is indicated for the treatment of infant botulism caused by toxin type A or B in patients below one year of age.
Dosing
- For Intravenous Use Only
Preparation for Administration
- Botulism Immune Globulin does not contain a preservative. After reconstitution of the lyophilized product, the vial should be entered only once for the purpose of administration, and the infusion should begin within 2 hours of reconstitution.
- Remove the tab portion of the vial cap and clean the rubber stopper with 70% alcohol or equivalent.
- Reconstitute the lyophilized powder with 2 mL of Sterile Water for Injection USP, to obtain a 50 mg/mL Botulism Immune Globulin solution. A double-ended transfer needle or large syringe is suitable for adding the water for reconstitution. When using a double-ended transfer needle, insert one end first into the vial of water. The lyophilized powder is supplied in an evacuated vial; therefore, the water should transfer by suction (the jet of water should be aimed to the side of the vial). After the water is transferred into the evacuated vial, the residual vacuum should be released to hasten the dissolution.
- Rotate the container gently to wet all the powder. An approximately 30-minute interval should be allowed for dissolving the powder. DO NOT SHAKE THE VIAL, AS THIS WILL CAUSE FOAMING.
- Inspect Botulism Immune Globulin visually for particulate matter and discoloration prior to administration. Infuse the solution only if it is colorless, free of particulate matter, and not turbid.
- To prevent the transmission of hepatitis viruses or other infectious agents from one person to another, use sterile disposable syringes and needles. Never reuse syringes and needles.
Treatment of Infant Botulism Caused by Toxin Type A or B
- The recommended total dosage of Botulism Immune Globulin is 1.0 mL/kg (50 mg/kg), given as a single intravenous infusion as soon as the clinical diagnosis of infant botulism is made. Botulism Immune Globulin should be used with caution in patients with pre-existing renal insufficiency and in patients judged to be at increased risk of developing renal insufficiency (including, but not limited to, those with diabetes mellitus, volume depletion, paraproteinemia, sepsis, or who are receiving known nephrotoxic drugs).
Administration
- Do not pre-dilute Botulism Immune Globulin before infusion.
- Begin infusion within 2 hours after reconstitution is complete and conclude within 4 hours of reconstitution, unless infusion is temporarily interrupted for adverse reaction. Monitor vital signs continuously during infusion.
- Administer Botulism Immune Globulin intravenously using low volume tubing and a constant infusion pump (i.e., an IVAC pump or equivalent) through a separate intravenous line. If a separate line is not possible, it may be "piggybacked" into a pre-existing line if that line contains either Sodium Chloride Injection USP, or one of the following dextrose solutions (with or without NaCl added): 2.5% dextrose in water, 5% dextrose in water, 10% dextrose in water, or 20% dextrose in water. If a pre-existing line must be used, do not dilute Botulism Immune Globulin more than 1:2 with any of the above-named solutions. Admixtures of Botulism Immune Globulin with any other solutions have not been evaluated. Use an in-line or syringe-tip sterile, disposable filter (18 μm) for the administration of Botulism Immune Globulin.
- In the absence of prospective data allowing identification of the maximum safe dose, concentration, and rate of infusion in these patients, DO NOT EXCEED THE RECOMMENDED DOSE, CONCENTRATION, AND RATE OF INFUSION.
- Begin infusion slowly. Administer Botulism Immune Globulin intravenously at 0.5 mL per kg body weight per hour (25 mg/kg/h). If no untoward reactions occur after 15 minutes, the rate may be increased to 1.0 mL/kg/h (50 mg/kg/h). DO NOT EXCEED THIS RATE OF ADMINISTRATION. Monitor the patient closely during and after each rate change. At the recommended rates, infusion of the indicated dose should take 97.5 minutes total elapsed time.

- As adverse reactions experienced by patients treated with immune globulin intravenous (human) (IGIV) products have been related to the infusion rate, if the patient develops a minor side effect (i.e., flushing), slow the rate of infusion or temporarily interrupt the infusion. If anaphylaxis or a significant drop in blood pressure occurs, discontinue the infusion and administer epinephrine.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Botulinum antitoxin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Botulinum antitoxin in pediatric patients.
Contraindications
- As with other immunoglobulin preparations, Botulism Immune Globulin should not be used in individuals with a prior history of severe reaction to other human immunoglobulin preparations.
- Individuals with selective immunoglobulin A deficiency have the potential for developing antibodies to immunoglobulin A and could have anaphylactic reactions to the subsequent administration of blood products that contain immunoglobulin A.
Warnings
- Only administer Botulism Immune Globulin as an intravenous infusion, since other routes of administration have not been evaluated. Do not use Botulism Immune Globulin if the reconstituted solution is turbid
Patient Monitoring for Administration
- Patients should be well hydrated prior to the initiation of the Botulism Immune Globulin infusion.
- Assess renal function, including the measurement of blood urea nitrogen (BUN) or serum creatinine prior to the initial infusion of Botulism Immune Globulin . Periodic monitoring of renal function tests and urine output is particularly important in patients judged to have a potential risk for developing acute renal failure.[1-4] Increases in serum creatinine and BUN have been observed as soon as one to two days following treatment with other IGIV products.
- During administration, monitor the patient's vital signs continuously and observe the patient carefully for any associated symptoms.
- DO NOT EXCEED THE RECOMMENDED INFUSION RATE of 1 mL/kg/hour (50 mg/kg/h), and follow the infusion schedule closely . If a patient develops an infusion reaction, slow the rate of infusion immediately or temporarily interrupt the infusion.
Renal Adverse Reactions
- Other IGIV products have been reported to be associated with renal dysfunction, acute renal failure, osmotic nephrosis, and death. While these reports of renal dysfunction and acute renal failure have been associated with the use of many licensed IGIV products, those that contained sucrose as a stabilizer and were administered at daily doses of 400 mg/kg or greater have accounted for a disproportionate share of the total number. Botulism Immune Globulin contains sucrose as a stabilizer. Patients predisposed to acute renal failure include those patients with any degree of pre-existing renal insufficiency, diabetes mellitus, volume depletion, sepsis, paraproteinemia, or who are receiving known nephrotoxic drugs. Especially in such patients, Botulism Immune Globulin should be administered at the minimum concentration available and at the minimum rate of infusion practicable.
Transmission of Blood-Borne Infectious Agents
- Botulism Immune Globulin is made from human plasma and, like other plasma products, carries the possibility for transmission of blood-borne viral agents and, theoretically, the Creutzfeldt-Jakob disease agent. The risk of transmission of recognized blood-borne viruses has been reduced by screening plasma donors for prior exposure to certain viruses, for the presence of certain viral infections, and by the viral inactivation and/or removal properties of the precipitation procedures used for the purification of Botulism Immune Globulin . Despite these measures, some as yet unrecognized blood-borne infectious agents may not be inactivated by the manufacturing process; therefore, Botulism Immune Globulin, like any other blood product, should be given only if a benefit is expected .
Anaphylaxis
- Severe reactions, such as angioedema and anaphylactic shock, although not observed during clinical trials with Botulism Immune Globulin, are a possibility. Clinical anaphylaxis may occur even when the patient is not known to be sensitive to immune globulin products. A reaction may be related to the rate of infusion; therefore, carefully adhere to the infusion rates as outlined under . If anaphylaxis or a drop in blood pressure occurs, discontinue the infusion and administer epinephrine.
Although acute systemic allergic reactions were not seen in clinical trials with Botulism Immune Globulin, epinephrine should be available for treatment of acute allergic symptoms . If hypotension or anaphylaxis occurs, discontinue the administration of Botulism Immune Globulin immediately and give supportive care as needed.
Aseptic Meningitis Syndrome
- An aseptic meningitis syndrome (AMS) has been reported to occur infrequently in association with IGIV administration. The syndrome usually begins within several hours to two days following IGIV treatment. It is characterized by symptoms and signs that include the following: severe headache, nuchal rigidity, drowsiness, fever, photophobia, painful eye movements, and nausea and vomiting. Cerebrospinal fluid studies are frequently positive with pleocytosis up to several thousand cells per cubic millimeter, predominately from the granulocytic series, and with elevated protein levels up to several hundred mg/dL. Conduct a thorough neurological examination in patients exhibiting such symptoms and signs to rule out other causes of meningitis. AMS may occur more frequently in association with high total doses (2 g/kg) of IGIV treatment. Discontinuation of IGIV treatment has resulted in remission of AMS within several days without sequelae. AMS was not observed in clinical trials of Botulism Immune Globulin.
Hyperproteinemia, Hyponatremia, and Serum Viscosity
- Hyperproteinemia, hyponatremia, and increased serum viscosity have been observed following administration of IGIV products. It is clinically critical to distinguish true hyponatremia from pseudohyponatremia caused by decreased calculated serum osmolality or elevated osmolar gap, because treatment aimed at decreasing serum free water in patients with pseudohyponatremia may lead to volume depletion, a further increase in serum viscosity and a higher risk of thromboembolic events. These adverse events have not been observed with Botulism Immune Globulin.
Thrombotic Events
- Thrombotic events may occur following IGIV treatment. Patients at risk may include those with a history of atherosclerosis, multiple cardiovascular risk factors, advanced age, impaired cardiac output, coagulation disorders, prolonged periods of immobilization, and/or known or suspected hyperviscosity. Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies. For patients judged to be at risk of developing thrombotic events, administer Botulism Immune Globulin at the minimum rate of infusion practicable.
Hemolytic Anemia
- IGIV products may contain blood group antibodies, which can act as hemolysins and induce in vivo coating of red blood cells with immunoglobulin, causing a positive direct antiglobulin reaction and, rarely, hemolysis. Hemolytic anemia may develop subsequent to IGIV therapy due to enhanced red blood cell sequestration.
- Monitor patients for clinical signs and symptoms of hemolysis. If these are present after Botulism Immune Globulin infusion, perform appropriate confirmatory laboratory testing.
Transfusion-Related Acute Lung Injury (TRALI)
- Non-cardiogenic pulmonary edema may occur in patients following IGIV treatment. TRALI is characterized by severe respiratory distress, pulmonary edema, hypoxemia, normal left ventricular function, and fever. Symptoms typically occur within 1 to 6 hours following treatment.
- Monitor patients for pulmonary adverse reactions. If TRALI is suspected, perform appropriate tests for the presence of anti-neutrophil antibodies in both the product and patient serum.
- TRALI may be managed using oxygen therapy with adequate ventilatory support.
Precautions
Adverse Reactions
Clinical Trials Experience
- Serious adverse reactions were not observed in clinical trials using Botulism Immune Globulin.
- The most common adverse reaction observed with Botulism Immune Globulin treatment during clinical trials (>5%) was skin rash.
- Other reactions such as chills, muscle cramps, back pain, fever, nausea, vomiting, and wheezing were the most frequent adverse reactions observed during the clinical trials of similarly-prepared human IGIV products. The incidence of these reactions was less than 5% of all infusions in Botulism Immune Globulin clinical trials, and these reactions were most often related to infusion rates.
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- Two clinical studies of Botulism Immune Globulin were performed: (1) an adequate and well-controlled study to evaluate safety and efficacy of Botulism Immune Globulin, which used Botulism Immune Globulin Lot 1, and (2) an open label study to collect additional safety data and confirm efficacy, which used Botulism Immune Globulin Lot 2. Different methodologies were used to collect adverse events in the controlled study and open label study. Minor clinical events that were not recorded as adverse events in the controlled study were recorded as adverse events in the open label study
- The only adverse event considered possibly related to Botulism Immune Globulin administration was a mild, transient erythematous rash of the face or trunk. The following table summarizes the occurrence of rash by day of study relative to day of treatment for the randomized, controlled clinical trial (RCT) and for the open label study (OLS).

- In the controlled study, when only treatment emergent events are considered, 14% of the Botulism Immune Globulin-treated patients experienced erythematous rash during or after study infusion. Eight percent of placebo-treated patients also experienced erythematous rash in this study. A similar rash is known to occur both in infant botulism patients who have not received any IGIV products[16] and in patients treated with other IGIVs,[2,3] making it difficult to ascertain the causality of the rash.
- In the controlled study only, the following adverse events occurred in at least 5% of the patients receiving Botulism Immune Globulin or placebo:


- Adverse event coding was used in the open label study to distinguish between minor clinical events that required no intervention and more significant events that required intervention. For example, "increased blood pressure" or "decreased blood pressure" was assigned when transient changes in blood pressure were observed, whereas "hypertension" or "hypotension" was assigned when more prolonged or significant changes were observed.
Postmarketing Experience
- Because postmarketing reporting of adverse reactions is voluntary and from a population of uncertain size, it is not always possible to reliably estimate the frequency of these reactions or establish a causal relationship to product exposure.
- Experience with Botulism Immune Globulin. No adverse reactions have been identified or reported that are ascribed to the use of Botulism Immune Globulin during postapproval use. Retrospective publications have shown safety-related information consistent with the safety-related information in the approved product labeling, and no new safety-related information has been presented for Botulism Immune Globulin.
- Experience with Other IGIV Products. Some classes of adverse reactions that have not been reported in Botulism Immune Globulin clinical studies or postmarketing experience have been observed with the overall post-approval use of other IGIV products, as shown in the following table.

Drug Interactions
- Admixtures of Botulism Immune Globulin with other drugs have not been evaluated. It is recommended that Botulism Immune Globulin be administered separately from other drugs or medications that the patient may be receiving .
- Antibodies present in immune globulin preparations may interfere with the immune response to live virus vaccines such as polio, measles, mumps, and rubella; THEREFORE, VACCINATION WITH LIVE VIRUS VACCINES SHOULD BE DEFERRED UNTIL APPROXIMATELY THREE OR MORE MONTHS AFTER ADMINISTRATION OF Botulism Immune Globulin. If such vaccinations were given shortly before or after Botulism Immune Globulin administration, revaccination may be necessary.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Botulinum antitoxin in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Botulinum antitoxin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Botulinum antitoxin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Botulinum antitoxin with respect to nursing mothers.
Pediatric Use
- Botulism Immune Globulin has been studied for safety and efficacy only in patients below one year of age. It has not been tested in other populations.
Geriatic Use
There is no FDA guidance on the use of Botulinum antitoxin with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Botulinum antitoxin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Botulinum antitoxin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Botulinum antitoxin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Botulinum antitoxin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Botulinum antitoxin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Botulinum antitoxin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
There is limited information regarding Monitoring of Botulinum antitoxin in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Botulinum antitoxin in the drug label.
Overdosage
- Although limited data are available, clinical experience with other immunoglobulin preparations suggests that the major manifestations would be those related to volume overload
Pharmacology
There is limited information regarding Botulinum antitoxin Pharmacology in the drug label.
Mechanism of Action
- Botulism Immune Globulin contains IgG antibodies from the immunized donors who contributed to the plasma pool from which the product was derived. The titer of antibodies in the reconstituted product against type A botulinum toxin is at least 15 IU/mL and against type B toxin is at least 4.0 IU/mL. For toxin types A and B, by definition, 1 IU of botulinum antitoxin neutralizes 10,000 intraperitoneal mouse LD50 of botulinum toxin. The titers of antibody against botulinum neurotoxins C, D, and E have not been determined. In the case of infants who may be exposed to botulinum neurotoxin type A or B, this product is expected to provide the relevant antibodies at levels sufficient to neutralize the expected levels of circulating neurotoxin.
- Botulism Immune Globulin contains antibodies specific for botulinum neurotoxin types A and B that bind to and neutralize circulating toxin types A and B in the patient.
Structure
- Botulism Immune Globulin, Botulism Immune Globulin Intravenous (Human) (BIG-IV), is a solvent-detergent-treated, sterile, lyophilized powder of immunoglobulin G (IgG), stabilized with 5% sucrose and 1% albumin (human). It contains no preservative. The purified immunoglobulin is derived from pooled adult plasma from persons who were immunized with pentavalent botulinum toxoid and selected for their high titers of neutralizing antibody against botulinum neurotoxins type A and B. All donors were tested and their sera found to be negative for antibodies against the human immunodeficiency virus and the hepatitis B and hepatitis C viruses.
- The pooled plasma was fractionated by cold ethanol precipitation of the proteins according to the Cohn/Oncley method, modified to yield a product suitable for intravenous administration. Several steps in the manufacturing process have been validated for their ability to inactivate or remove viruses that may not have been detected in the Source Plasma.
- These include Cohn/Oncley fractionation (Fraction I through Supernatant III Filtrate); nanofiltration through one 75-nm and two 35-nm filters; and solvent/detergent viral inactivation. These viral reduction steps have been validated in a series of in vitro experiments for their capacity to inactivate and/or remove Human Immunodeficiency Virus type 1 (HIV-1) and the following model viruses: bovine viral diarrhea virus (BVDV) as a model for hepatitis C virus; mouse encephalomyelitis virus (MEMV) as a model for hepatitis A virus; and pseudorabies virus (PRV), feline calicivirus (FCV), and Sindbis virus to cover a wide range of physicochemical properties in the model viruses studied. Total mean log10reductions range from 4.63 to greater than 16 log10 as shown in the following table.

- Additional testing performed with bovine parvovirus (as a model for parvovirus B19) showed a mean cumulative reduction factor of greater than 7.34 log10 for Cohn/Oncley fractionation and solvent/detergent treatment followed by hydrophobic chromatography. A mean cumulative reduction factor of 2.55 log10 was observed for removal of porcine parvovirus by nanofiltration.
- When reconstituted with Sterile Water for Injection USP, each cubic centimeter (milliliter) contains approximately 50 ± 10 mg immunoglobulin, primarily IgG, and trace amounts of IgA and IgM; 50 mg sucrose; 10 mg albumin (human); and approximately 20 × 10-3 mEq sodium. The reconstituted solution should appear colorless and translucent
Pharmacodynamics
- Formal studies on pharmacodynamics have not been conducted with Botulism Immune Globulin.
Pharmacokinetics
- Traditional pharmacokinetic studies of Botulism Immune Globulin have not been performed. However, the following table summarizes the mean serum titer of the anti-A component of Botulism Immune Globulin following administration.

- The half-life of injected Botulism Immune Globulin has been shown to be approximately 28 days in infants,[14] which is in agreement with existing data for other immunoglobulin preparations.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Botulinum antitoxin in the drug label.
Clinical Studies
- Two clinical studies in infant botulism were performed: (1) an adequate and well-controlled study to evaluate the safety and efficacy of Botulism Immune Globulin (N=129), and (2) an open label study to collect additional safety data and confirm efficacy (N=293). In the adequate and well-controlled clinical study, Botulism Immune Globulin, given within the first 3 days of hospital admission to 59 patients with laboratory-confirmed infant botulism, has been shown to reduce the following:
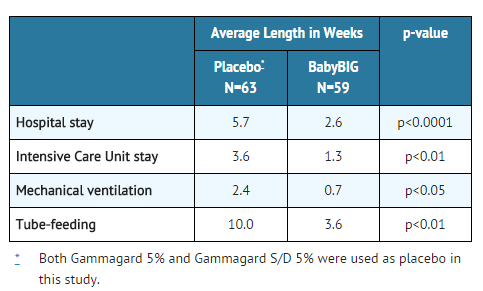
- Length of hospital stay was also analyzed by patient age in both the adequate and well-controlled study and in an open label study.

- The observed reduction in length of hospital stay was statistically significant (p<0.01) with the exception of the 0 to 60-day age stratum, where small patient numbers limited the statistical power.
- Length of hospital stay was analyzed in the adequate and well-controlled study by race (white versus non-white):

- Length of hospital stay was significantly reduced in both white and non-white patients (p=0.002).
- Botulism Immune Globulin has not been tested for safety and efficacy in adults.
How Supplied
- NDC 68403-1100-6, 100 mg ± 20 mg lyophilized immunoglobulin single-dose vial individually packaged in a carton, supplied with 2 mL Sterile Water for Injection USP for reconstitution.
- Store the vial containing the lyophilized product between 2° and 8°C (35.6° to 46.4°F). Do not store Botulism Immune Globulin in the reconstituted state.
- Use reconstituted Botulism Immune Globulin within 2 hours.
- Do not use beyond expiration date, and dispose unused product in accordance with local requirements.
Storage
There is limited information regarding Botulinum antitoxin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Botulinum antitoxin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Botulinum antitoxin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Discuss the risks and benefits of Botulism Immune Globulin use with the patient's legal guardians, including the possibility of adverse reactions, e.g., hypersensitivity reactions such as anaphylaxis, as well as aseptic meningitis, TRALI, hemolysis, renal failure, and thrombosis .
- Inform patient's legal guardians that Botulism Immune Globulin is made from human plasma and may contain infectious agents that can cause disease. While the risk of transmitting an infection has been reduced by screening plasma donors for prior exposure, testing donated plasma, and inactivating or removing certain viruses during manufacturing, the patient's guardian should report any symptoms that concern them .
- Inform patient's legal guardians that Botulism Immune Globulin may interfere with immune response to live viral vaccines (e.g., MMR) and instruct them to notify the healthcare provider of this potential interaction when the patient is to receive vaccinations
Precautions with Alcohol
- Alcohol-Botulinum antitoxin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- BabyBIG
Look-Alike Drug Names
There is limited information regarding Botulinum antitoxin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Botulinum antitoxin
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Botulinum antitoxin |Label Name=Botulinum antitoxin11.png
}}
{{#subobject:
|Label Page=Botulinum antitoxin |Label Name=Botulinum antitoxin12.png
}}
{{#subobject:
|Label Page=Botulinum antitoxin |Label Name=Botulinum antitoxin13.png
}}