Neurofibroma pathophysiology
|
Neurofibroma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Neurofibroma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Neurofibroma pathophysiology |
|
Risk calculators and risk factors for Neurofibroma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]; Associate Editor(s)-in-Chief: Sara Mohsin, M.D.[3]Shanshan Cen, M.D. [4]
Overview
Neurofibromas arise from the nonmyelinating-type Schwann cells. Gene involved in the pathogenesis of plexiform neurofibroma is NF1 which codes for neurofibromin that leads to loss of RAS control causing increased activity of downstream RAS pathways involved in increased cell growth and survival. Neurofibromas can occur anywhere in body. On gross pathology, a nonencapsulated superficial mass is the characteristic finding of localised or diffuse neurofibroma; whereas the "bag of worms" appearance is the characteristic finding of plexiform neurofibroma. On microscopic histopathological analysis, nerve fibers, schwann cells, spindle cells with wavy nuclei without pleomorphism, shredded carrot collagen, moderate increase of cellularity vis-a-vis normal dermis, blood vessels, mast cells, pseudomeissnerian bodies, and varying degrees of myxoid degeneration are characteristic findings of neurofibroma. Electron microscopy of neurofibromas shows Schwann cells enclosing axons in plasmalemmal invaginations (mesaxons).
Pathogenesis
- Neurofibromas arise from the nonmyelinating-type Schwann cells[1][2][3]
- Plexiform neurofibromas can grow from nerves in the skin or from more internal nerve bundles, and can be very large[4]
- About 10% of plexiform neurofibromas undergo transformation into a malignant peripheral nerve sheath tumor (MPNST)[5]
- In 2006, Yang et al demonstrated a critical neurofibroma microenvironment interaction that includes SCF-stimulated Nf1+/− mast cells potentiating Nf1+/− fibroblast functions[6]
- Nearly one-half of the dry tumor weight is made up of collagen secreted by fibroblasts, which comprise the major cellular portion of neurofibroma
- Fibroblasts migrate, proliferate, and synthesize collagen in response to transforming growth factor β (TGF-β)
- Fibroblast bioactivity is TGF-β dependent
- Nf1+/− mast cell is the critical effector in the paracrine induction of neurofibroma pathogenesis
- TGF-β–dependent Nf1+/− fibroblast hyperactivity is a result of increased kinase activity of c-abl secondary to increased Ras-GTP
 |
Genetics
- Gene involved in the pathogenesis of plexiform neurofibroma is NF1.[7]
- The NF1 gene is composed of 60 exons spanning 350kb of genomic data, and maps to chromosomal region 17q11.2.[8] This gene codes for neurofibromin which is a large 220-250 KDa cytoplasmic protein that is composed of 2,818 amino acids with three alternatively spliced exons (9a, 23a, and 48a) in the encoding gene. The functional part of neurofibromin is GAP, or GTPase-activating protein. GAP accelerates the conversion of the active GTP-bound RAS to its inactive GDP-bound form, inactivating RAS and reducing RAS-mediated growth signaling. Loss of RAS control leads to increased activity of other signaling pathways including RAF, ERK1/2, PI3K, PAK, MAPK, SCF/c-kit and mTOR-S6 kinase. It is suspected that this increased activity of downstream RAS pathways might work together to increase cell growth and survival.[9][10]
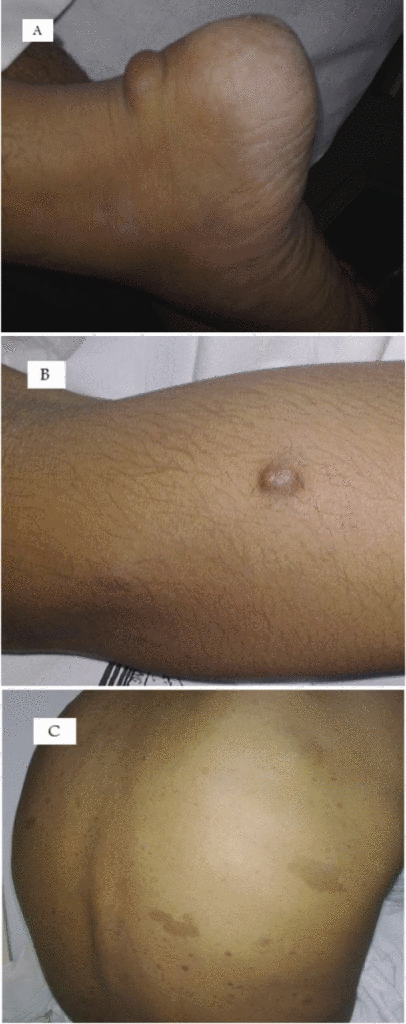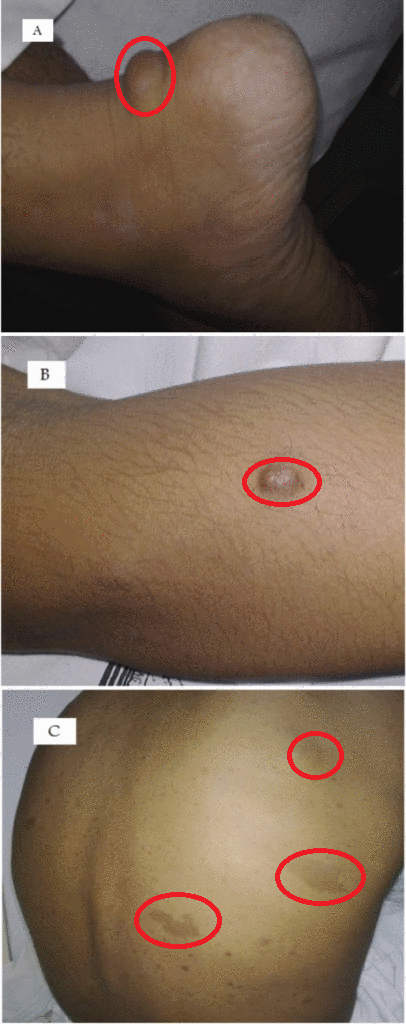
Gross Pathology
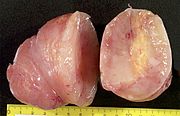
Neurofibromas can occur anywhere in body. Following are the gross features of different types of neurofibromas:
Localised neurofibroma and Diffuse neurofibroma
- Superficial mass[7]
- Not encapsulated
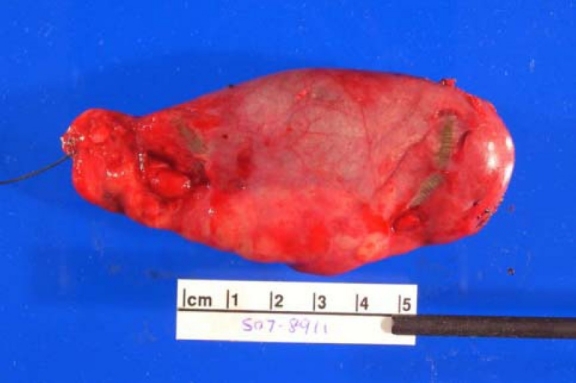
Soft tissue neurofibroma
- Not encapsulated, softer (more gelatinous) than schwannoma
- Superficial tumors are small, pedunculated nodules protruding from skin (molluscum pendulum)
- Deeper tumors are larger, may cause tortuous enlargement of peripheral nerves (plexiform neurofibromas)
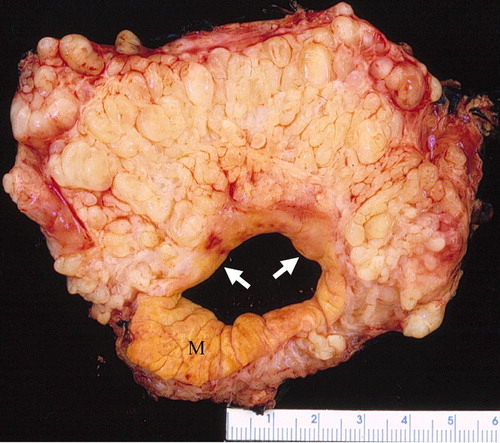
Plexiform neurofibroma
- "Bag of worms" appearance[11][12][13]
- Associated with grossly enlarged and tortuous nerves
- Deep tumors are often large
- Highly vascularized and locally invasive
 |
 |
 |
 |
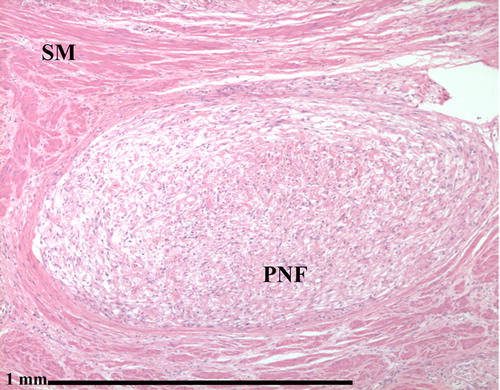
Microscopic Pathology
- Made of nerve fibers, schwann cells (cells that cover the nerve fibers), blood vessels, inflammatory white blood cells (mast cells), and connective tissue (fibroblasts and loose material called extracellular matrix)
- Multiple enlarged fascicles of randomly oriented thin spindled nerve sheath cells are embeded in a stromal mucin and wire-like collageneous matrix within fibrous connective tissue[2]
- Spindle cells have hyperchromatic, elongated, wavy, buckled, small and bland nuclei with indistinct scant cytoplasm[14]
- No pleomorphism
- Tactile-like bodies are also detected
- Varying degrees of myxoid degeneration
- Unencapsulated
- Moderate increase of cellularity vis-a-vis normal dermis
- Uniphasic, low to moderate cellularity (generally hypocellular)
- Occasional mast cells,[3][15] and lymphocytes, rare foam cells
- Thin and thick collagen strands (“shredded carrot collagen”)
- Nerve fibers run through a neurofibroma (making excision more difficult with increased likelihood of nerve damage)
- Nerve frequently not grossly identifiable
- Features of specific differentiation (e.g. pseudomeissnerian bodies) may be present occassionally
- Low mitotic index
- No clear division between Antoni A and B areas within the tumor
- No well formed verocy bodies
- No epithelial component
- No distinct lobulation
- Seldom cystic
- Cellularity and organization are generally insufficient to produce palisading
Neurofibroma with Degenerative Atypia ("Ancient change")
- Localized hyperchromatic atypical cells ("ancient change") with:
- Large, pleomorphic nuclei
- Cytoplasmic intranuclear inclusions
- Smudgy chromatin
- Inconspicuous nucleoli
- Absent or very low mitotic activity
- Low to moderate cellularity
- Often large, long standing lesions
Soft tissue neurofibromas
- Proliferation of all elements of peripheral nerves
- Schwann cells with wire like collagen fibrils (wavy serpentine nuclei, pointed ends), stromal mucosubstances, mast cells, Wagner-Meissner corpuscles, Pacinian corpuscles, axons (highlight with silver or acetylcholinesterase stain, NSE, neurofilament), fibroblasts and collagen
- Perineurial cells in plexiform types, mitotic figures are rare
- Less of a fascicular pattern than fibromatosis
- May be infiltrative, have myxoid areas, contain melanin pigment, have epitheloid morphology
- Rarely has skeletal differentiation (neuromuscular hamartoma)
- No Verocay bodies
- No nuclear palisading
- No hyalinized thickening of vessel walls
Plexiform neurofibromas
- Nodular or diffuse
- Diffuse cases are also known as elephantiasis neurofibromatosa; characterized by an overgrowth of epidermal and subcutaneous tissue
- Hypocellular with myxoid background; contains Schwann cells, fibroblasts and mast cells
- Occasional nuclear palisading
- Rarely is pigmented due to melanocytes
- No biphasic pattern of schwannoma
- Target sign on histopathology represents a central core made up of collagenous and fibrillary tissue with peripheral less densely cellular myxoid tissue[16]
 |
 |
 |
 |
Electron microscopy
- Schwann cells enclose axons in plasmalemmal invaginations (mesaxons)
References
- ↑ Muir D, Neubauer D, Lim IT, Yachnis AT, Wallace MR. (2003). "Tumorigenic properties of neurofibromin-deficient neurofibroma Schwann cells". American Journal of Pathology. 158 (2): 501–13. doi:10.1016/S0002-9440(10)63992-2. PMC 1850316. PMID 11159187.
- ↑ 2.0 2.1 Bernthal, NM.; Jones, KB.; Monument, MJ.; Liu, T.; Viskochil, D.; Randall, RL. (2013). "Lost in translation: ambiguity in nerve sheath tumor nomenclature and its resultant treatment effect". Cancers (Basel). 5 (2): 519–28. doi:10.3390/cancers5020519. PMID 24216989.
- ↑ 3.0 3.1 Staser, K.; Yang, FC.; Clapp, DW. (2010). "Mast cells and the neurofibroma microenvironment". Blood. 116 (2): 157–64. doi:10.1182/blood-2009-09-242875. PMID 20233971. Unknown parameter
|month=ignored (help) - ↑ Neurofibroma. Wikipedia 2015. https://en.wikipedia.org/wiki/Neurofibroma#cite_note-Yamashiroya2002-3 Accessed on November 17, 2015
- ↑ Mautner VF, Friedrich RE, von Deimling A, Hagel C, Korf B, Knöfel MT, Wenzel R, Fünsterer C. (2003). "Malignant peripheral nerve sheath tumours in neurofibromatosis type 1: MRI supports the diagnosis of malignant plexiform neurofibroma". American Journal of Pathology. 45 (9): 618–25. doi:10.1007/s00234-003-0964-6. PMID 12898075.
- ↑ http://www.bloodjournal.org/content/116/2/157?sso-checked=true
- ↑ 7.0 7.1 Neurofibroma. Dr Bruno Di Muzio and Dr Maxime St-Amant et al. Radiopaedia.org 2015. http://radiopaedia.org/articles/neurofibroma. Accessed on November 17, 2015
- ↑ MH Shen, PS Harper, M Upadhyaya. (1996). "Molecular genetics of neurofibromatosis type 1 (NF1)". Journal of Medical Genetics. 33 (1): 2–17. doi:10.1136/jmg.33.1.2. PMC 1051805. PMID 8825042.
- ↑ Rubin JB, Gutmann DH. (2005). "Neurofibromatosis type 1 - a model for nervous system tumour formation?". Nature Reviews Cancer. 5 (7): 557–64. doi:10.1038/nrc1653. PMID 16069817.
- ↑ Johnson MR, Look AT, DeClue JE, Valentine MB, Lowy DR. (1993). "Inactivation of the NF1 gene in human melanoma and neuroblastoma cell lines without impaired regulation of GTP.Ras". Proceedings of the National Academy of Sciences of the USA. 90 (12): 5539–43. doi:10.1073/pnas.90.12.5539. PMC 46756. PMID 8516298.
- ↑ Wilkinson LM, Manson D, Smith CR (2004). "Best cases from the AFIP: plexiform neurofibroma of the bladder". Radiographics : a Review Publication of the Radiological Society of North America, Inc. 24 Suppl 1: S237–42. doi:10.1148/rg.24si035170. PMID 15486243. Retrieved 2015-11-13.
- ↑ Neurofibroma. Libre Pathology 2015. http://librepathology.org/wiki/index.php/Neurofibroma#cite_note-pmid15486243-2 Accessed on November 17, 2015
- ↑ Neurofibroma. Libre Pathology 2015. http://librepathology.org/wiki/index.php/Neurofibroma#cite_note-pmid15486243-2 Accessed on November 17, 2015
- ↑ http://surgpathcriteria.stanford.edu/peripheral-nerve/neurofibroma/
- ↑ Neurofibroma. Libre Pathology 2015. http://librepathology.org/wiki/index.php/Neurofibroma#cite_note-pmid15486243-2 Accessed on November 17, 2015
- ↑ https://pubs.rsna.org/doi/10.1148/rg.24si035170#REF8