Captopril: Difference between revisions
No edit summary |
No edit summary |
||
| Line 141: | Line 141: | ||
|warnings=====Anaphylactoid and Possibly Related Reactions==== | |warnings=====Anaphylactoid and Possibly Related Reactions==== | ||
Presumably because angiotensin-converting enzyme inhibitors affect the metabolism of eicosanoids and polypeptides, including endogenous bradykinin, patients receiving ACE inhibitors (including captopril) may be subject to a variety of adverse reactions, some of them serious. | Presumably because [[angiotensin-converting enzyme inhibitors]] affect the metabolism of [[eicosanoids]] and [[polypeptides]], including endogenous [[bradykinin]], patients receiving [[ACE inhibitors]] (including captopril) may be subject to a variety of adverse reactions, some of them serious. | ||
*'''Head and Neck Angioedema''': Angioedema involving the extremities, face, lips, mucous membranes, tongue, glottis or larynx has been seen in patients treated with ACE inhibitors, including captopril. If angioedema involves the tongue, glottis or larynx, airway obstruction may occur and be fatal. Emergency therapy, including but not necessarily limited to, subcutaneous administration of a 1:1000 solution of epinephrine should be promptly instituted. | *'''Head and Neck Angioedema''': [[Angioedema]] involving the [[extremities]], [[face]], [[lips]], [[mucous membranes]], [[tongue]], [[glottis]] or [[larynx]] has been seen in patients treated with [[ACE inhibitors]], including captopril. If [[angioedema]] involves the [[tongue]], [[glottis]] or [[larynx]], airway obstruction may occur and be fatal. Emergency therapy, including but not necessarily limited to, subcutaneous administration of a 1:1000 solution of [[epinephrine]] should be promptly instituted. | ||
*Swelling confined to the face, mucous membranes of the mouth, lips and extremities has usually resolved with discontinuation of captopril; some cases required medical therapy. | *Swelling confined to the face, [[mucous membranes]] of the mouth, [[lips]] and extremities has usually resolved with discontinuation of captopril; some cases required medical therapy. | ||
*'''Intestinal Angioedema''': Intestinal angioedema has been reported in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); in some cases there was no prior history of facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor. Intestinal angioedema should be included in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain. | *'''Intestinal Angioedema''': Intestinal [[angioedema]] has been reported in patients treated with [[ACE inhibitors]]. These patients presented with abdominal pain (with or without [[nausea]] or [[vomiting]]); in some cases there was no prior history of facial [[angioedema]] and [[C-1 esterase]] levels were normal. The [[angioedema]] was diagnosed by procedures including [[abdominal CT]] scan or [[ultrasound]], or at surgery, and symptoms resolved after stopping the [[ACE inhibitor]]. Intestinal [[angioedema]] should be included in the differential diagnosis of patients on [[ACE inhibitors]] presenting with [[abdominal pain]]. | ||
*'''Anaphylactoid reactions during desensitization''': Two patients undergoing desensitizing treatment with hymenoptera venom while receiving ACE inhibitors sustained life-threatening anaphylactoid | *'''Anaphylactoid reactions during desensitization''': Two patients undergoing desensitizing treatment with [[hymenoptera]] [[venom]] while receiving [[ACE inhibitors]] sustained life-threatening [[anaphylactoid reaction]]s. In the same patients, these reactions were avoided when [[ACE inhibitors]] were temporarily withheld, but they reappeared upon inadvertent rechallenge. | ||
*'''Anaphylactoid reactions during membrane exposure:''' Anaphylactoid | *'''Anaphylactoid reactions during membrane exposure:''' [[Anaphylactoid reaction]]s have been reported in patients dialyzed with high-flux membranes and treated concomitantly with an [[ACE inhibitor]]. [[Anaphylactoid reaction]]s have also been reported in patients undergoing [[low-density lipoprotein]] apheresis with [[dextran sulfate]] absorption. | ||
====Neutropenia/Agranulocytosis==== | ====Neutropenia/Agranulocytosis==== | ||
*Neutropenia (<1000/mm3) with myeloid hypoplasia has resulted from use of captopril. About half of the neutropenic patients developed systemic or oral cavity | *[[Neutropenia]] (<1000/mm3) with [[myeloid hypoplasia]] has resulted from use of captopril. About half of the [[neutropenic]] patients developed systemic or [[oral cavity infection]]s or other features of the syndrome of [[agranulocytosis]]. | ||
*The risk of neutropenia is dependent on the clinical status of the patient | *The risk of [[neutropenia]] is dependent on the clinical status of the patient. | ||
*In clinical trials in patients with hypertension who have normal renal function (serum creatinine less than 1.6 mg/dL and no collagen vascular disease), neutropenia has been seen in one patient out of over 8,600 exposed. | *In clinical trials in patients with [[hypertension]] who have normal renal function ([[serum creatinine]] less than 1.6 mg/dL and no [[collagen vascular disease]]), [[neutropenia]] has been seen in one patient out of over 8,600 exposed. | ||
*In patients with some degree of renal failure (serum creatinine at least 1.6 mg/dL) but no collagen vascular disease, the risk of neutropenia in clinical trials was about 1 per 500, a frequency over 15 times that for uncomplicated hypertension. Daily doses of captopril were relatively high in these patients, particularly in view of their diminished renal function. In foreign marketing experience in patients with renal failure, use of allopurinol concomitantly with captopril has been associated with neutropenia but this association has not appeared in U.S. reports. | *In patients with some degree of [[renal failure]] (serum [[creatinine]] at least 1.6 mg/dL) but no collagen vascular disease, the risk of [[neutropenia]] in clinical trials was about 1 per 500, a frequency over 15 times that for uncomplicated [[hypertension]]. Daily doses of captopril were relatively high in these patients, particularly in view of their diminished [[renal function]]. In foreign marketing experience in patients with [[renal failure]], use of [[allopurinol]] concomitantly with captopril has been associated with [[neutropenia]] but this association has not appeared in U.S. reports. | ||
*In patients with collagen vascular | *In patients with [[collagen vascular disease]]s (e.g., [[systemic lupus erythematosus]], [[scleroderma]]) and impaired [[renal function]], [[neutropenia]] occurred in 3.7 percent of patients in clinical trials. | ||
*While none of the over 750 patients in formal clinical trials of heart failure developed neutropenia, it has occurred during the subsequent clinical experience. About half of the reported cases had serum creatinine ≥1.6 mg/dL and more than 75 percent were in patients also receiving procainamide. In heart failure, it appears that the same risk factors for neutropenia are present. | *While none of the over 750 patients in formal clinical trials of heart failure developed [[neutropenia]], it has occurred during the subsequent clinical experience. About half of the reported cases had serum [[creatinine]] ≥1.6 mg/dL and more than 75 percent were in patients also receiving [[procainamide]]. In heart failure, it appears that the same risk factors for [[neutropenia]] are present. | ||
*The neutropenia has usually been detected within three months after captopril was started. Bone marrow examinations in patients with neutropenia consistently showed myeloid hypoplasia, frequently accompanied by erythroid hypoplasia and decreased numbers of megakaryocytes (e.g., hypoplastic bone marrow and pancytopenia); anemia and thrombocytopenia were sometimes seen. | *The [[neutropenia]] has usually been detected within three months after captopril was started. Bone marrow examinations in patients with [[neutropenia]] consistently showed [[myeloid hypoplasia]], frequently accompanied by [[erythroid hypoplasia]] and decreased numbers of [[megakaryocytes]] (e.g., hypoplastic [[bone marrow]] and [[pancytopenia]]); [[anemia]] and [[thrombocytopenia]] were sometimes seen. | ||
*In general, neutrophils returned to normal in about two weeks after captopril was discontinued, and serious infections were limited to clinically complex patients. About 13 percent of the cases of neutropenia have ended fatally, but almost all fatalities were in patients with serious illness, having collagen vascular disease, renal failure, heart failure or immunosuppressant therapy, or a combination of these complicating factors. | *In general, [[neutrophils ] returned to normal in about two weeks after captopril was discontinued, and serious infections were limited to clinically complex patients. About 13 percent of the cases of [[neutropenia]] have ended fatally, but almost all fatalities were in patients with serious illness, having [[collagen vascular disease]], [[renal failure]], [[heart failure]] or [[immunosuppressant]] therapy, or a combination of these complicating factors. | ||
====Evaluation of the hypertensive or heart failure patient should always include assessment of renal function==== | ====Evaluation of the hypertensive or heart failure patient should always include assessment of renal function==== | ||
| Line 347: | Line 347: | ||
====Agents Having Vasodilator Activity==== | ====Agents Having Vasodilator Activity==== | ||
* Data on the effect of concomitant use of other [[vasodilators]] in patients receiving captopril for [[heart failure]] are not available; therefore, [[nitroglycerin]] or other [[nitrates]] (as used for management of [[angina]]) or other drugs having vasodilator activity should, if possible, be discontinued before starting captopril. If resumed during captopril therapy, such agents should be administered cautiously, and perhaps at lower dosage. | |||
====Agents Causing Renin Release==== | ====Agents Causing Renin Release==== | ||
| Line 359: | Line 359: | ||
====Agents Increasing Serum Potassium==== | ====Agents Increasing Serum Potassium==== | ||
* Since captopril decreases [[aldosterone]] production, elevation of serum [[potassium]] may occur. [[Potassium-sparing diuretics]] such as [[spironolactone]], [[triamterene]], or [[amiloride]], or [[potassium]] supplements should be given only for documented [[hypokalemia]], and then with caution, since they may lead to a significant increase of serum [[potassium]]. Salt substitutes containing [[potassium]] should also be used with caution. | |||
====Lithium==== | ====Lithium==== | ||
Revision as of 14:46, 1 July 2014
{{DrugProjectFormSinglePage |authorTag=Ahmed Zaghw, M.D. [1], Amr Marawan, M.D. [2] |genericName=captopril |aOrAn=an |drugClass=Angiontensin converting enzyme inhibitor |indication=hypertension, heart failure, left ventricular dysfunction after myocardial infarction, diabetic nephropathy |hasBlackBoxWarning=Yes |adverseReactions=hypotension, rash, hyperkalemia (11% ), disorder of taste, cough (0.5% to 2%) |blackBoxWarningTitle=Fetal Toxicity |blackBoxWarningBody=When pregnancy is detected, discontinue captopril as soon as possible.
Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. |fdaLIADAdult======Hypertension=====
- Dosing Information
- Initial dose: Captopril 25 mg PO bid or Captopril 25 mg PO tid ; may be increased after 1 to 2 weeks to Captopril 50 mg PO bid or Captopril 50 mg PO tid , then to Captopril 100 to 150 mg PO bid/tid (in combination with a thiazide diuretic) if needed (MAX 450 mg daily)
Captopril tablets, USP are indicated for the treatment of hypertension.
In using captopril, consideration should be given to the risk of neutropenia/agranulocytosis.
Captopril may be used as initial therapy for patients with normal renal function, in whom the risk is relatively low. In patients with impaired renal function, particularly those with collagen vascular disease, captopril should be reserved for hypertensives who have either developed unacceptable side effects on other drugs, or have failed to respond satisfactorily to drug combinations.
Captopril is effective alone and in combination with other antihypertensive agents, especially thiazide-type diuretics. The blood pressure lowering effects of captopril and thiazides are approximately additive.
Heart Failure
- Dosing Information
- Initial dose: (patients with normal or low blood pressure, vigorous diuretic therapy, volume depletion) Captopril 6.25 to 12.5 mg PO tid
- Initial dose: Captopril PO 25 mg tid
- Maintenance dose: Captopril 50 to 100 mg PO tid (MAX 450 mg PO daily)
Captopril tablets, USP are indicated in the treatment of congestive heart failure usually in combination with diuretics and digitalis. The beneficial effect of captopril in heart failure does not require the presence of digitalis, however, most controlled clinical trial experience with captopril has been in patients receiving digitalis, as well as diuretic treatment.
Left Ventricular Dysfunction After Myocardial Infarction
- Dosing Information
- Initial dose: Captopril 6.25 mg PO for one dose starting as early as 3 days after myocardial infarction,
- Maintenance dose: Captopril 12.5 mg tid a day increased to 25 mg PO tid a day in several days; target dose 50 mg PO tid over the next several weeks as tolerated
Captopril tablets, USP are indicated to improve survival following myocardial infarction in clinically stable patients with left ventricular dysfunction manifested as an ejection fraction ≤40% and to reduce the incidence of overt heart failure and subsequent hospitalizations for congestive heart failure in these patients.
Diabetic Nephropathy
- Dosing Information
- (type 1 diabetes mellitus) Captopril 25 mg PO tid
- (type 2 diabetes mellitus) Captopril 12.5 mg bid , increased to Captopril 12.5 mg tid after 3 months
Captopril tablets, USP are indicated for the treatment of diabetic nephropathy (proteinuria >500 mg/day) in patients with type I insulin-dependent diabetes mellitus and retinopathy. Captopril decreases the rate of progression of renal insufficiency and development of serious adverse clinical outcomes (death or need for renal transplantation or dialysis).
In considering use of captopril, it should be noted that in controlled trials ACE inhibitors have an effect on blood pressure that is less in black patients than in non-blacks. In addition, ACE inhibitors (for which adequate data are available) cause a higher rate of angioedema in black than in non-black patients. |offLabelAdultGuideSupport======Acute ST segment elevation myocardial infarction=====
- Class of Recommendation: Class I
- Level of Evidence: Level A
- Dosing Information
- An angiotensin-converting enzyme (ACE) inhibitor should be administered within the first 24 hours to all patients with STEMI with anterior location, HF, or ejection fraction (EF) less than or equal to 0.40, unless contraindicated.[1]
|offLabelAdultNoGuideSupport======Cystinuria=====
- Dosing information
- Case report 1: 150 mg/day for 26 weeks followed by 75 mg for 9 weeks[2]
- Case report 2: 25 mg bid (titrated to achieve a concentration of urinary cystine of less than 200 mg/liter, or to a maximum of 150 mg/day)[3]
Prophylaxis of diabetic retinopathy
- Dosing information
- Case report: 12.5 mg bid for 3 months followed by 12.5 mg tid[4]
Erythrocytosis
- Dosing information
- 25 mg qd [5]This beneficial effect may not be sustained.
Non-diabetic kidney disease
- Dosing information
- RCT report:
- Initial dosage: 12.5 mg bid
- Followed-up treatment: increase the dosage bi-weekly
- Final aim: either blood pressure normalization or reach the maximum of 50 mg bid [6]
- Research 1
- Dosage for the first month: 6.25 mg/day
- Dosage for the second month: titrated to 12.5 mg/day
- Finally dosage: titrated to 25 mg/day[7]
- Research 2
- Initial dosage: 6.25 mg tid
- Followed-up treatment: 25 mg tid[8]
- Case report: 25 mg/day, max: 75 mg/day[9]
Diagnosis of primary aldosteronism
- Dosing information
- 25 mg/day[10]
Pseudoprimary hyperaldosteronism
- Dosing information
Renovascular Hypertension
- Dosing information
- As an alternative therapy in patients with contraindications to surgery or renal artery angioplasty.
|fdaLIADPed=FDA Package Insert for captopril contains no information regarding safety and efficacy in pediatric patients |offLabelPedGuideSupport=There is limited information about the guideline-supported off-label use.
|offLabelPedNoGuideSupport=
Chronic Heart Failure(infants)
- Dosing Information
- 4 mg/kg via nasogastric tube[13]
Kidney Imaging
- Dosing Information
- 0.2-0.4 mg/kg PO qd[14]
|contraindications=* Hypersensitivity to this product or any other angiotensin-converting enzyme inhibitor
- History of angioedema during therapy with any other ACE inhibitor)
|warnings=====Anaphylactoid and Possibly Related Reactions====
Presumably because angiotensin-converting enzyme inhibitors affect the metabolism of eicosanoids and polypeptides, including endogenous bradykinin, patients receiving ACE inhibitors (including captopril) may be subject to a variety of adverse reactions, some of them serious.
- Head and Neck Angioedema: Angioedema involving the extremities, face, lips, mucous membranes, tongue, glottis or larynx has been seen in patients treated with ACE inhibitors, including captopril. If angioedema involves the tongue, glottis or larynx, airway obstruction may occur and be fatal. Emergency therapy, including but not necessarily limited to, subcutaneous administration of a 1:1000 solution of epinephrine should be promptly instituted.
- Swelling confined to the face, mucous membranes of the mouth, lips and extremities has usually resolved with discontinuation of captopril; some cases required medical therapy.
- Intestinal Angioedema: Intestinal angioedema has been reported in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); in some cases there was no prior history of facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor. Intestinal angioedema should be included in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain.
- Anaphylactoid reactions during desensitization: Two patients undergoing desensitizing treatment with hymenoptera venom while receiving ACE inhibitors sustained life-threatening anaphylactoid reactions. In the same patients, these reactions were avoided when ACE inhibitors were temporarily withheld, but they reappeared upon inadvertent rechallenge.
- Anaphylactoid reactions during membrane exposure: Anaphylactoid reactions have been reported in patients dialyzed with high-flux membranes and treated concomitantly with an ACE inhibitor. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein apheresis with dextran sulfate absorption.
Neutropenia/Agranulocytosis
- Neutropenia (<1000/mm3) with myeloid hypoplasia has resulted from use of captopril. About half of the neutropenic patients developed systemic or oral cavity infections or other features of the syndrome of agranulocytosis.
- The risk of neutropenia is dependent on the clinical status of the patient.
- In clinical trials in patients with hypertension who have normal renal function (serum creatinine less than 1.6 mg/dL and no collagen vascular disease), neutropenia has been seen in one patient out of over 8,600 exposed.
- In patients with some degree of renal failure (serum creatinine at least 1.6 mg/dL) but no collagen vascular disease, the risk of neutropenia in clinical trials was about 1 per 500, a frequency over 15 times that for uncomplicated hypertension. Daily doses of captopril were relatively high in these patients, particularly in view of their diminished renal function. In foreign marketing experience in patients with renal failure, use of allopurinol concomitantly with captopril has been associated with neutropenia but this association has not appeared in U.S. reports.
- In patients with collagen vascular diseases (e.g., systemic lupus erythematosus, scleroderma) and impaired renal function, neutropenia occurred in 3.7 percent of patients in clinical trials.
- While none of the over 750 patients in formal clinical trials of heart failure developed neutropenia, it has occurred during the subsequent clinical experience. About half of the reported cases had serum creatinine ≥1.6 mg/dL and more than 75 percent were in patients also receiving procainamide. In heart failure, it appears that the same risk factors for neutropenia are present.
- The neutropenia has usually been detected within three months after captopril was started. Bone marrow examinations in patients with neutropenia consistently showed myeloid hypoplasia, frequently accompanied by erythroid hypoplasia and decreased numbers of megakaryocytes (e.g., hypoplastic bone marrow and pancytopenia); anemia and thrombocytopenia were sometimes seen.
- In general, [[neutrophils ] returned to normal in about two weeks after captopril was discontinued, and serious infections were limited to clinically complex patients. About 13 percent of the cases of neutropenia have ended fatally, but almost all fatalities were in patients with serious illness, having collagen vascular disease, renal failure, heart failure or immunosuppressant therapy, or a combination of these complicating factors.
Evaluation of the hypertensive or heart failure patient should always include assessment of renal function
- If captopril is used in patients with impaired renal function, white blood cell and differential counts should be evaluated prior to starting treatment and at approximately two-week intervals for about three months, then periodically.
- In patients with collagen vascular disease or who are exposed to other drugs known to affect the white cells or immune response, particularly when there is impaired renal function, captopril should be used only after an assessment of benefit and risk, and then with caution.
- All patients treated with captopril should be told to report any signs of infection (e.g., sore throat, fever). If infection is suspected, white cell counts should be performed without delay.
- Since discontinuation of captopril and other drugs has generally led to prompt return of the white count to normal, upon confirmation of neutropenia (neutrophil count < 1000/mm3) the physician should withdraw captopril and closely follow the patient's course.
Proteinuria
- Total urinary proteins greater than 1 g per day were seen in about 0.7 percent of patients receiving captopril.
- About 90 percent of affected patients had evidence of prior renal disease or received relatively high doses of captopril (in excess of 150 mg/day), or both.
- The nephrotic syndrome occurred in about one-fifth of proteinuric patients.
- In most cases, proteinuria subsided or cleared within six months whether or not captopril was continued.
- Parameters of renal function, such as BUN and creatinine, were seldom altered in the patients with proteinuria.
Hypotension
- Excessive hypotension was rarely seen in hypertensive patients but is a possible consequence of captopril use in salt/volume depleted persons (such as those treated vigorously with diuretics), patients with heart failure or those patients undergoing renal dialysis.
- In heart failure, where the blood pressure was either normal or low, transient decreases in mean blood pressure greater than 20 percent were recorded in about half of the patients. This transient hypotension is more likely to occur after any of the first several doses and is usually well tolerated, producing either no symptoms or brief mild lightheadedness, although in rare instances it has been associated with arrhythmia or conduction defects. Hypotension was the reason for discontinuation of drug in 3.6 percent of patients with heart failure.
- BECAUSE OF THE POTENTIAL FALL IN BLOOD PRESSURE IN THESE PATIENTS, THERAPY SHOULD BE STARTED UNDER VERY CLOSE MEDICAL SUPERVISION. A starting dose of 6.25 or 12.5 mg t.i.d. may minimize the hypotensive effect. Patients should be followed closely for the first two weeks of treatment and whenever the dose of captopril and/or diuretic is increased. In patients with heart failure, reducing the dose of diuretic, if feasible, may minimize the fall in blood pressure.
- Hypotension is not per se a reason to discontinue captopril. Some decrease of systemic blood pressure is a common and desirable observation upon initiation of captopril tablets, USP treatment in heart failure. The magnitude of the decrease is greatest early in the course of treatment; this effect stabilizes within a week or two, and generally returns to pretreatment levels, without a decrease in therapeutic efficacy, within two months.
Hepatic Failure
Rarely, ACE inhibitors have been associated with a syndrome that starts with cholestatic jaundice and progresses to fulminant hepatic necrosis and (sometimes) death. The mechanism of this syndrome is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevations of hepatic enzymes should discontinue the ACE inhibitor and receive appropriate medical follow-up. |clinicalTrials=Reported incidences are based on clinical trials involving approximately 7000 patients.
Renal
- About one of 100 patients developed proteinuria.
- Each of the following has been reported in approximately 1 to 2 of 1000 patients and are of uncertain relationship to drug use: renal insufficiency, renal failure, nephrotic syndrome, polyuria, oliguria, and urinary frequency.
Hematologic
- Neutropenia/agranulocytosis has occurred. Cases of anemia, thrombocytopenia, and pancytopenia have been reported.
Dermatologic
- Rash, often with pruritus, and sometimes with fever, arthralgia, and eosinophilia, occurred in about 4 to 7 (depending on renal status and dose) of 100 patients, usually during the first four weeks of therapy. It is usually maculopapular, and rarely urticarial. The rash is usually mild and disappears within a few days of dosage reduction, short-term treatment with an antihistamine agent, and/or discontinuing therapy; remission may occur even if captopril is continued. Pruritus, without rash, occurs in about 2 of 100 patients. Between 7 and 10 percent of patients with skin rash have shown an eosinophilia and/or positive ANA titers. A reversible associated pemphigoid-like lesion, and photosensitivity, have also been reported.
Cardiovascular
- Hypotension may occur; for discussion of hypotension with captopril therapy.
- Tachycardia, chest pain, and palpitations have each been observed in approximately 1 of 100 patients.
- Angina pectoris, myocardial infarction, Raynaud's syndrome, and congestive heart failure have each occurred in 2 to 3 of 1000 patients.
Dysgeusia
- Approximately 2 to 4 (depending on renal status and dose) of 100 patients developed a diminution or loss of taste perception. Taste impairment is reversible and usually self-limited (2 to 3 months) even with continued drug administration. Weight loss may be associated with the loss of taste.
Angioedema
- Angioedema involving the extremities, face, lips, mucous membranes, tongue, glottis or larynx has been reported in approximately one in 1000 patients. Angioedema involving the upper airways has caused fatal airway obstruction.
Cough
- Cough has been reported in 0.5 to 2% of patients treated with captopril in clinical trials.
The following have been reported in about 0.5 to 2 percent of patients but did not appear at increased frequency compared to placebo or other treatments used in controlled trials:
- Gastric irritation
- Abdominal pain
- Nausea
- Vomiting
- Diarrhea
- Anorexia
- Constipation
- Aphthous ulcers
- Peptic ulcer
- Dizziness
- Headache
- Malaise
- Fatigue
- Insomnia
- Dry mouth
- Dyspnea
- Alopecia
- Paresthesias
|postmarketing=====Body As A Whole====
General
Cardiovascular
- Cardiac arrest, cerebrovascular accident/insufficiency, rhythm disturbances, orthostatic hypotension, syncope.
Dermatologic
- Bullous pemphigoid, erythema multiforme (including Stevens-Johnson syndrome), exfoliative dermatitis.
Gastrointestinal
Hematologic
Hepatobiliary
- Jaundice, hepatitis, including rare cases of necrosis, cholestasis.
Metabolic
- Symptomatic hyponatremia.
Musculoskeletal
Nervous/Psychiatric
Respiratory
Special Senses
Urogenital
- As with other ACE inhibitors, a syndrome has been reported which may include: fever, myalgia, arthralgia, interstitial nephritis, vasculitis, rash or other dermatologic manifestations, eosinophilia and an elevated ESR.
Altered Laboratory Findings
Serum Electrolytes
- Hyperkalemia
- Small increases in serum potassium, especially in patients with renal impairment.
- Hyponatremia
- Particularly in patients receiving a low sodium diet or concomitant diuretics.
BUN/Serum Creatinine
- Transient elevations of BUN or serum creatinine especially in volume or salt depleted patients or those with renovascular hypertension may occur. Rapid reduction of longstanding or markedly elevated blood pressure can result in decreases in the glomerular filtration rate and, in turn, lead to increases in BUN or serum creatinine.
Hematologic
- A positive ANA has been reported.
Liver Function Tests
- Elevations of liver transaminases, alkaline phosphatase, and serum bilirubin have occurred.
|drugInteractions=====Dual Blockade of the Renin-Angiotensin System (RAS)====
- Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Closely monitor blood pressure, renal function and electrolytes in patients on captopril and other agents that affect the RAS.
- Do not co-administer aliskiren with captopril in patients with diabetes. Avoid use of aliskiren with captopril in patients with renal impairment (GFR <60 ml/min).
Non-Steroidal Anti-Inflammatory Agents including Selective Cyclooxygenase – 2 Inhibitors (COX-2 Inhibitors)
- In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co-administration of NSAIDs, including selective COX-2 inhibitors, with ACE inhibitors, including captopril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving captopril and NSAID therapy. The antihypertensive effect of ACE inhibitors, including captopril, may be attenuated by NSAIDs.
Hypotension - Patients On Diuretic Therapy
- Patients on diuretics and especially those in whom diuretic therapy was recently instituted, as well as those on severe dietary salt restriction or dialysis, may occasionally experience a precipitous reduction of blood pressure usually within the first hour after receiving the initial dose of captopril.
- The possibility of hypotensive effects with captopril can be minimized by either discontinuing the diuretic or increasing the salt intake approximately one week prior to initiation of treatment with captopril tablets, USP or initiating therapy with small doses (6.25 or 12.5 mg). Alternatively, provide medical supervision for at least one hour after the initial dose. If hypotension occurs, the patient should be placed in a supine position and, if necessary, receive an intravenous infusion of normal saline. This transient hypotensive response is not a contraindication to further doses which can be given without difficulty once the blood pressure has increased after volume expansion.
Agents Having Vasodilator Activity
- Data on the effect of concomitant use of other vasodilators in patients receiving captopril for heart failure are not available; therefore, nitroglycerin or other nitrates (as used for management of angina) or other drugs having vasodilator activity should, if possible, be discontinued before starting captopril. If resumed during captopril therapy, such agents should be administered cautiously, and perhaps at lower dosage.
Agents Causing Renin Release
- Captopril's effect will be augmented by antihypertensive agents that cause renin release. For example, diuretics (e.g., thiazides) may activate the renin-angiotensin-aldosterone system.
Agents Affecting Sympathetic Activity
- The sympathetic nervous system may be especially important in supporting blood pressure in patients receiving captopril alone or with diuretics. Therefore, agents affecting sympathetic activity (e.g., ganglionic blocking agents or adrenergic neuron blocking agents) should be used with caution. Beta-adrenergic blocking drugs add some further antihypertensive effect to captopril, but the overall response is less than additive.
Agents Increasing Serum Potassium
- Since captopril decreases aldosterone production, elevation of serum potassium may occur. Potassium-sparing diuretics such as spironolactone, triamterene, or amiloride, or potassium supplements should be given only for documented hypokalemia, and then with caution, since they may lead to a significant increase of serum potassium. Salt substitutes containing potassium should also be used with caution.
Lithium
- Increased serum lithium levels and symptoms of lithium toxicity have been reported in patients receiving concomitant lithium and ACE inhibitor therapy. These drugs should be coadministered with caution and frequent monitoring of serum lithium levels is recommended. If a diuretic is also used, it may increase the risk of lithium toxicity.
Cardiac Glycosides
- In a study of young healthy male subjects no evidence of a direct pharmacokinetic captopril-digoxin interaction could be found.
Loop Diuretics
- Furosemide administered concurrently with captopril does not alter the pharmacokinetics of captopril in renal impaired hypertensive patients.
Allopurinol
- In a study of healthy male volunteers no significant pharmacokinetic interaction occurred when captopril and allopurinol were administered concomitantly for 6 days.
Gold
- Nitritoid reactions (symptoms include facial flushing, nausea, vomiting and hypotension) have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant ACE inhibitor therapy including captopril.
Drug /Laboratory Test Interaction
- Captopril may cause a false-positive urine test for acetone.
|FDAPregCat=D |useInPregnancyFDA=Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue captopril as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mothers and fetus.
In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue, captopril unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to captopril for hypotension, oliguria, and hyperkalemia.
When captopril was given to rabbits at doses about 0.8 to 70 times (on a mg/kg basis) the maximum recommended human dose, low incidences of craniofacial malformations were seen. No teratogenic effects of captopril were seen in studies of pregnant rats and hamsters. On a mg/kg basis, the doses used were up to 150 times (in hamsters) and 625 times (in rats) the maximum recommended human dose. |AUSPregCat=D |useInPregnancyAUS=Drugs which have caused, are suspected to have caused, or may be expected to cause an increased incidence of human fetal malformations or irreversible damage. These drugs may also have adverse pharmacological effects. Accompanying texts should be consulted for further details. |useInLaborDelivery=:*FDA Package Insert for captopril contains no information regarding Labor and Delivery. |useInNursing=:*Concentrations of captopril in human milk are approximately one percent of those in maternal blood. Because of the potential for serious adverse reactions in nursing infants from captopril, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of captopril to the mother. |useInPed=:*If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function. While captopril may be removed from the adult circulation by hemodialysis, there is inadequate data concerning the effectiveness of hemodialysis for removing it from the circulation of neonates or children Peritoneal dialysis is not effective for removing captopril; there is no information concerning exchange transfusion for removing captopril form the general circulation.
- Safety and effectiveness in pediatric patients have not been established. There is limited experience reported in the literature with the use of captopril in the pediatric population; dosage, on a weight basis, was generally reported to be comparable to or less than that used in adults.
- Infants, especially newborns, may be more susceptible to the adverse hemodynamic effects of captopril. Excessive, prolonged and unpredictable decreases in blood pressure and associated complications, including oliguria and seizures, have been reported.
- Captopril should be used in pediatric patients only if other measures for controlling blood pressure have not been effective.
|useInGeri=:*FDA Package Insert for Captopril contains no information regarding the drug in Geriatrics. |useInGender=:*FDA Package Insert for Captopril contains no information regarding the use of the drug and Gender. |useInRace=:*FDA Package Insert for Captopril contains no information regarding the Race. |useInRenalImpair======Dosage Adjustment in Renal Impairment=====
- Because captopril is excreted primarily by the kidneys, excretion rates are reduced in patients with impaired renal function. These patients will take longer to reach steady-state captopril levels and will reach higher steady-state levels for a given daily dose than patients with normal renal function. Therefore, these patients may respond to smaller or less frequent doses.
- Accordingly, for patients with significant renal impairment, initial daily dosage of captopril should be reduced, and smaller increments utilized for titration, which should be quite slow (one- to two-week intervals). After the desired therapeutic effect has been achieved, the dose should be slowly back-titrated to determine the minimal effective dose. When concomitant diuretic therapy is required, a loop diuretic (e.g., furosemide), rather than a thiazide diuretic, is preferred in patients with severe renal impairment.
Animal Toxicology
- Chronic oral toxicity studies were conducted in rats (2 years), dogs (47 weeks; 1 year), mice (2 years), and monkeys (1 year). Significant drug-related toxicity included effects on hematopoiesis, renal toxicity, erosion/ulceration of the stomach, and variation of retinal blood vessels.
- Reductions in hemoglobin and/or hematocrit values were seen in mice, rats, and monkeys at doses 50 to 150 times the maximum recommended human dose (MRHD) of 450 mg, assuming a 50-kg subject. On a body-surface-area basis, these doses are 5 to 25 times maximum recommended dose (MRHD). Anemia, leukopenia, thrombocytopenia, and bone marrow suppression occurred in dogs at doses 8 to 30 times MRHD on a body-weight basis (4 to 15 times MRHD on a surface-area basis). The reductions in hemoglobin and hematocrit values in rats and mice were only significant at 1 year and returned to normal with continued dosing by the end of the study. Marked anemia was seen at all dose levels (8 to 30 times MRHD) in dogs, whereas moderate to marked leukopenia was noted only at 15 and 30 times MRHD and thrombocytopenia at 30 times MRHD. The anemia could be reversed upon discontinuation of dosing. Bone marrow suppression occurred to a varying degree, being associated only with dogs that died or were sacrificed in a moribund condition in the 1 year study. However, in the 47-week study at a dose 30 times MRHD, bone marrow suppression was found to be reversible upon continued drug administration.
- Captopril caused hyperplasia of the juxtaglomerular apparatus of the kidneys in mice and rats at doses 7 to 200 times MRHD on a body-weight basis (0.6 to 35 times MRHD on a surface-area basis); in monkeys at 20 to 60 times MRHD on a body-weight basis (7 to 20 times MRHD on a surface-area basis); and in dogs at 30 times MRHD on a body-weight basis (15 times MRHD on a surface-area basis).
- Gastric erosions/ulcerations were increased in incidence in male rats at 20 to 200 times MRHD on a body-weight basis (3.5 and 35 times MRHD on a surface-area basis); in dogs at 30 times MRHD on a body-weight basis (15 times on MRHD on a surface-area basis); and in monkeys at 65 times MRHD on a body-weight basis (20 times MRHD on a surface-area basis). Rabbits developed gastric and intestinal ulcers when given oral doses approximately 30 times MRHD on a body-weight basis (10 times MRHD on a surface-area basis) for only 5 to 7 days.
- In the two-year rat study, irreversible and progressive variations in the caliber of retinal vessels (focal sacculations and constrictions) occurred at all dose levels (7 to 200 times MRHD) on a body-weight basis; 1 to 35 times MRHD on a surface-area basis in a dose-related fashion. The effect was first observed in the 88th week of dosing, with a progressively increased incidence thereafter, even after cessation of dosing.
|useInHepaticImpair=FDA Package Insert for Captopril contains no information regarding Hepatic Impairment. |useInReproPotential=:*FDA Package Insert for Captopril contains no information regarding Females of Reproductive Potential and Males. |useInImmunocomp=:*FDA Package Insert for Captopril contains no information regarding Immunocompromised Patients. |administration=Captopril should be taken one hour before meals. Dosage must be individualized.
Hypertension
Initiation of therapy requires consideration of recent antihypertensive drug treatment, the extent of blood pressure elevation, salt restriction, and other clinical circumstances. If possible, discontinue the patient's previous antihypertensive drug regimen for one week before starting captopril. The initial dose of captopril tablets, USP is 25 mg b.i.d. or t.i.d. If satisfactory reduction of blood pressure has not been achieved after one or two weeks, the dose may be increased to 50 mg b.i.d. or t.i.d. Concomitant sodium restriction may be beneficial when captopril is used alone. The dose of captopril in hypertension usually does not exceed 50 mg t.i.d. Therefore, if the blood pressure has not been satisfactorily controlled after one to two weeks at this dose, (and the patient is not already receiving a diuretic), a modest dose of a thiazide-type diuretic (e.g., hydrochlorothiazide, 25 mg daily), should be added. The diuretic dose may be increased at one- to two-week intervals until its highest usual antihypertensive dose is reached. If captopril is being started in a patient already receiving a diuretic, captopril therapy should be initiated under close medical supervision (see WARNINGS and PRECAUTIONS, Drug Interactions regarding hypotension), with dosage and titration of captopril as noted above. If further blood pressure reduction is required, the dose of captopril may be increased to 100 mg b.i.d. or t.i.d. and then, if necessary, to 150 mg b.i.d. or t.i.d. (while continuing the diuretic). The usual dose range is 25 to 150 mg b.i.d. or t.i.d. A maximum daily dose of 450 mg captopril should not be exceeded. For patients with severe hypertension (e.g., accelerated or malignant hypertension), when temporary discontinuation of current antihypertensive therapy is not practical or desirable, or when prompt titration to more normotensive blood pressure levels is indicated, diuretic should be continued but other current antihypertensive medication stopped and captopril dosage promptly initiated at 25 mg b.i.d. or t.i.d., under close medical supervision. When necessitated by the patient's clinical condition, the daily dose of captopril may be increased every 24 hours or less under continuous medical supervision until a satisfactory blood pressure response is obtained or the maximum dose of captopril is reached. In this regimen, addition of a more potent diuretic, e.g., furosemide, may also be indicated. Beta-blockers may also be used in conjunction with captopril therapy (see PRECAUTIONS, Drug Interactions), but the effects of the two drugs are less than additive.
Heart Failure
Initiation of therapy requires consideration of recent diuretic therapy and the possibility of severe salt/volume depletion. In patients with either normal or low blood pressure, who have been vigorously treated with diuretics and who may be hyponatremic and/or hypovolemic, a starting dose of 6.25 or 12.5 mg t.i.d. may minimize the magnitude or duration of the hypotensive effect (see WARNINGS, Hypotension); for these patients, titration to the usual daily dosage can then occur within the next several days. For most patients the usual initial daily dosage is 25 mg t.i.d. After a dose of 50 mg t.i.d. is reached, further increases in dosage should be delayed, where possible, for at least two weeks to determine if a satisfactory response occurs. Most patients studied have had a satisfactory clinical improvement at 50 or 100 mg t.i.d. A maximum daily dose of 450 mg of captopril should not be exceeded. Captopril should generally be used in conjunction with a diuretic and digitalis. Captopril therapy must be initiated under very close medical supervision.
Left Ventricular Dysfunction After Myocardial Infarction
The recommended dose for long-term use in patients following a myocardial infarction is a target maintenance dose of 50 mg t.i.d. Therapy may be initiated as early as three days following a myocardial infarction. After a single dose of 6.25 mg, captopril therapy should be initiated at 12.5 mg t.i.d. Captopril should then be increased to 25 mg t.i.d. during the next several days and to a target dose of 50 mg t.i.d. over the next several weeks as tolerated (see CLINICAL PHARMACOLOGY). Captopril may be used in patients treated with other post-myocardial infarction therapies, e.g., thrombolytics, aspirin, beta blockers. Diabetic Nephropathy The recommended dose of captopril for long term use to treat diabetic nephropathy is 25 mg t.i.d. Other antihypertensives such as diuretics, beta blockers, centrally acting agents or vasodilators may be used in conjunction with captopril if additional therapy is required to further lower blood pressure.
Dosage Adjustment in Renal Impairment
Because captopril is excreted primarily by the kidneys, excretion rates are reduced in patients with impaired renal function. These patients will take longer to reach steady-state captopril levels and will reach higher steady-state levels for a given daily dose than patients with normal renal function. Therefore, these patients may respond to smaller or less frequent doses. Accordingly, for patients with significant renal impairment, initial daily dosage of captopril should be reduced, and smaller increments utilized for titration, which should be quite slow (one- to two-week intervals). After the desired therapeutic effect has been achieved, the dose should be slowly back-titrated to determine the minimal effective dose. When concomitant diuretic therapy is required, a loop diuretic (e.g., furosemide), rather than a thiazide diuretic, is preferred in patients with severe renal impairment. (See WARNINGS, Anaphylactoid reactions during membrane exposure and PRECAUTIONS, hemodialysis.) |monitoring=======Lithium======
Increased serum lithium levels and symptoms of lithium toxicity have been reported in patients receiving concomitant lithium and ACE inhibitor therapy. These drugs should be coadministered with caution and frequent monitoring of serum lithium levels is recommended. If a diuretic is also used, it may increase the risk of lithium toxicity. |IVCompat=FDA Package Insert for captopril contains no information regarding IV Compatibility. |overdose====Hypotension===
Signs and Symptoms
Correction of hypotension would be of primary concern.
Management
Volume expansion with an intravenous infusion of normal saline is the treatment of choice for restoration of blood pressure. While captopril may be removed from the adult circulation by hemodialysis, there is inadequate data concerning the effectiveness of hemodialysis for removing it from the circulation of neonates or children. Peritoneal dialysis is not effective for removing captopril; there is no information concerning exchange transfusion for removing captopril from the general circulation. |drugBox=
| |
| Template:Px | |
Captopril
| |
| Systematic (IUPAC) name | |
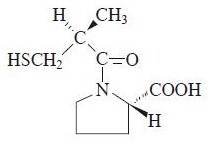
| (2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl] pyrrolidine-2-carboxylic acid | |
| Identifiers | |
| CAS number | |
| ATC code | C09 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 217.29 |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 70–75% |
| Metabolism | hepatic |
| Half life | 1.9 hours |
| Excretion | renal |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
Template:Unicode Prescription only |
| Routes | oral |
|mechAction=The mechanism of action of captopril has not yet been fully elucidated. Its beneficial effects in hypertension and heart failure appear to result primarily from suppression of the renin-angiotensin-aldosterone system. However, there is no consistent correlation between renin levels and response to the drug. Renin, an enzyme synthesized by the kidneys, is released into the circulation where it acts on a plasma globulin substrate to produce angiotensin I, a relatively inactive decapeptide. Angiotensin I is then converted by angiotensin converting enzyme (ACE) to angiotensin II, a potent endogenous vasoconstrictor substance. Angiotensin II also stimulates aldosterone secretion from the adrenal cortex, thereby contributing to sodium and fluid retention.
Captopril prevents the conversion of angiotensin I to angiotensin II by inhibition of ACE, a peptidyldipeptide carboxy hydrolase. This inhibition has been demonstrated in both healthy human subjects and in animals by showing that the elevation of blood pressure caused by exogenously administered angiotensin I was attenuated or abolished by captopril. In animal studies, captopril did not alter the pressor responses to a number of other agents, including angiotensin II and norepinephrine, indicating specificity of action.
ACE is identical to "bradykininase", and captopril may also interfere with the degradation of the vasodepressor peptide, bradykinin. Increased concentrations of bradykinin or prostaglandin E2 may also have a role in the therapeutic effect of captopril.
Inhibition of ACE results in decreased plasma angiotensin II and increased plasma renin activity (PRA), the latter resulting from loss of negative feedback on renin release caused by reduction in angiotensin II. The reduction of angiotensin II leads to decreased aldosterone secretion, and, as a result, small increases in serum potassium may occur along with sodium and fluid loss.
The antihypertensive effects persist for a longer period of time than does demonstrable inhibition of circulating ACE. It is not known whether the ACE present in vascular endothelium is inhibited longer than the ACE in circulating blood. |structure=Captopril is a specific competitive inhibitor of angiotensin I-converting enzyme (ACE), the enzyme responsible for the conversion of angiotensin I to angiotensin II. Captopril is designated chemically as 1-((2S)-3-mercapto-2-methylpropionyl)-L-proline (MW 217.29) and has the following structural formula:
Captopril is a white to off-white crystalline powder that may have a slight sulfurous odor; it is soluble in water (approx. 160 mg/mL), methanol, and ethanol and sparingly soluble in chloroform and ethyl acetate. Each captopril tablet, USP 12.5 mg, 25 mg, 50 mg and 100 mg for oral administration contains 12.5 mg, 25 mg, 50 mg and 100 mg, respectively. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide NF, croscarmellose sodium NF, lactose monohydrate NF, magnesium stearate NF and microcrystalline cellulose NF. |PD=After oral administration of therapeutic doses of captopril, rapid absorption occurs with peak blood levels at about one hour. The presence of food in the gastrointestinal tract reduces absorption by about 30 to 40 percent; captopril therefore should be given one hour before meals. Based on carbon-14 labeling, average minimal absorption is approximately 75 percent. In a 24-hour period, over 95 percent of the absorbed dose is eliminated in the urine; 40 to 50 percent is unchanged drug; most of the remainder is the disulfide dimer of captopril and captopril-cysteine disulfide.
Approximately 25 to 30 percent of the circulating drug is bound to plasma proteins. The apparent elimination half-life for total radioactivity in blood is probably less than 3 hours. An accurate determination of half-life of unchanged captopril is not, at present, possible, but it is probably less than 2 hours. In patients with renal impairment, however, retention of captopril occurs. |PK=After oral administration of therapeutic doses of captopril, rapid absorption occurs with peak blood levels at about one hour. The presence of food in the gastrointestinal tract reduces absorption by about 30 to 40 percent; captopril therefore should be given one hour before meals. Based on carbon-14 labeling, average minimal absorption is approximately 75 percent. In a 24-hour period, over 95 percent of the absorbed dose is eliminated in the urine; 40 to 50 percent is unchanged drug; most of the remainder is the disulfide dimer of captopril and captopril-cysteine disulfide.
Approximately 25 to 30 percent of the circulating drug is bound to plasma proteins. The apparent elimination half-life for total radioactivity in blood is probably less than 3 hours. An accurate determination of half-life of unchanged captopril is not, at present, possible, but it is probably less than 2 hours. In patients with renal impairment, however, retention of captopril occurs. |nonClinToxic=====Carcinogenesis, Mutagenesis, Impairment of Fertility====
- Two-year studies with doses of 50 to 1350 mg/kg/day in mice and rats failed to show any evidence of carcinogenic potential. The high dose in these studies is 150 times the maximum recommended human dose of 450 mg, assuming a 50-kg subject. On a body-surface-area basis, the high doses for mice and rats are 13 and 26 times the maximum recommended human dose, respectively.
- Studies in rats have revealed no impairment of fertility.
Animal Toxicology
- Chronic oral toxicity studies were conducted in rats (2 years), dogs (47 weeks; 1 year), mice (2 years), and monkeys (1 year). Significant drug-related toxicity included effects on hematopoiesis, renal toxicity, erosion/ulceration of the stomach, and variation of retinal blood vessels.
- Reductions in hemoglobin and/or hematocrit values were seen in mice, rats, and monkeys at doses 50 to 150 times the maximum recommended human dose (MRHD) of 450 mg, assuming a 50-kg subject. On a body-surface-area basis, these doses are 5 to 25 times maximum recommended dose (MRHD). Anemia, leukopenia, thrombocytopenia, and bone marrow suppression occurred in dogs at doses 8 to 30 times MRHD on a body-weight basis (4 to 15 times MRHD on a surface-area basis). The reductions in hemoglobin and hematocrit values in rats and mice were only significant at 1 year and returned to normal with continued dosing by the end of the study. Marked anemia was seen at all dose levels (8 to 30 times MRHD) in dogs, whereas moderate to marked leukopenia was noted only at 15 and 30 times MRHD and thrombocytopenia at 30 times MRHD. The anemia could be reversed upon discontinuation of dosing. Bone marrow suppression occurred to a varying degree, being associated only with dogs that died or were sacrificed in a moribund condition in the 1 year study. However, in the 47-week study at a dose 30 times MRHD, bone marrow suppression was found to be reversible upon continued drug administration.
- Captopril caused hyperplasia of the juxtaglomerular apparatus of the kidneys in mice and rats at doses 7 to 200 times MRHD on a body-weight basis (0.6 to 35 times MRHD on a surface-area basis); in monkeys at 20 to 60 times MRHD on a body-weight basis (7 to 20 times MRHD on a surface-area basis); and in dogs at 30 times MRHD on a body-weight basis (15 times MRHD on a surface-area basis).
- Gastric erosions/ulcerations were increased in incidence in male rats at 20 to 200 times MRHD on a body-weight basis (3.5 and 35 times MRHD on a surface-area basis); in dogs at 30 times MRHD on a body-weight basis (15 times on MRHD on a surface-area basis); and in monkeys at 65 times MRHD on a body-weight basis (20 times MRHD on a surface-area basis). Rabbits developed gastric and intestinal ulcers when given oral doses approximately 30 times MRHD on a body-weight basis (10 times MRHD on surface-area basis) for only 5 to 7 days.
- In the two-year rat study, irreversible and progressive variations in the caliber of retinal vessels (focal sacculations and constrictions) occurred at all dose levels (7 to 200 times MRHD) on a body-weight basis; 1 to 35 times MRHD on a surface-area basis in a dose-related fashion. The effect was first observed in the 88th week of dosing, with a progressively increased incidence thereafter, even after cessation of dosing.
|clinicalStudies======Myocardial infarction=====
Placebo controlled studies of 12 weeks duration in patients who did not respond adequately to diuretics and digitalis show no tolerance to beneficial effects on ETT, open studies, with exposure up to 18 months in some cases, also indicate that ETT benefit is maintained. Clinical improvement has been observed in some patients where acute hemodynamic effects were minimal.
The Survival and Ventricular Enlargement (SAVE) study was a multicenter, randomized, double-blind, placebo-controlled trial conducted in 2,231 patients (age 21 to 79 years) who survived the acute phase of myocardial infarction and did not have active ischemia. Patients had left ventricular dysfunction (LVD), defined as a resting left ventricular ejection fraction ≤40%, but at the time of randomization were not sufficiently symptomatic to require ACE inhibitor therapy for heart failure. About half of the patients had symptoms of heart failure in the past. Patients were given a test dose of 6.25 mg oral captopril and were randomized within 3 to 16 days post-infarction to receive either captopril or placebo in addition to conventional therapy. Captopril was initiated at 6.25 mg or 12.5 mg t.i.d. and after two weeks titrated to a target maintenance dose of 50 mg t.i.d. About 80% of patients were receiving the target dose at the end of the study. Patients were followed for a minimum of two years and for up to five years, with an average follow-up of 3.5 years.
Baseline blood pressure was 113/70 mmHg and 112/70 mmHg for the placebo and captopril groups, respectively. Blood pressure increased slightly in both treatment groups during the study and was somewhat lower in the captopril group (119/74 vs. 125/77 mmHg at 1 yr).
Therapy with captopril improved long-term survival and clinical outcomes compared to placebo. The risk reduction for all cause mortality was 19% (P=0.02) and for cardiovascular death was 21% (P=0.014). Captopril treated subjects had 22% (P=0.034) fewer first hospitalizations for heart failure. Compared to placebo, 22% fewer patients receiving captopril developed symptoms of overt heart failure. There was no significant difference between groups in total hospitalizations for all cause (2056 placebo; 2036 captopril).
Captopril was well tolerated in the presence of other therapies such as aspirin, beta blockers, nitrates, vasodilators, calcium antagonists and diuretics.
Retinopathy and Proteinuria
In a multicenter, double-blind, placebo controlled trial, 409 patients, age 18 to 49 of either gender, with or without hypertension, with type I (juvenile type, onset before age 30) insulin-dependent diabetes mellitus, retinopathy, proteinuria ≥500 mg per day and serum creatinine ≤ 2.5 mg/dL, were randomized to placebo or captopril (25 mg t.i.d.) and followed for up to 4.8 years (median 3 years). To achieve blood pressure control, additional antihypertensive agents (diuretics, beta blockers, centrally acting agents or vasodilators) were added as needed for patients in both groups.
The captopril group had a 51% reduction in risk of doubling of serum creatinine (P<0.01) and a 51% reduction in risk for the combined endpoint of end-stage renal disease (dialysis or transplantation) or death (P<0.01). captopril treatment resulted in a 30% reduction in urine protein excretion within the first 3 months (P<0.05), which was maintained throughout the trial. The captopril group had somewhat better blood pressure control than the placebo group, but the effects of captopril on renal function were greater than would be expected from the group differences in blood pressure reduction alone. Captopril was well tolerated in this patient population.
In two multicenter, double-blind, placebo controlled studies, a total of 235 normotensive patients with insulin-dependent diabetes mellitus, retinopathy and microalbuminuria (20 to 200 mcg/min) were randomized to placebo or captopril (50 mg b.i.d.) and followed for up to 2 years. Captopril delayed the progression to overt nephropathy (proteinuria ≥ 500 mg/day) in both studies (risk reduction 67% to 76%; P<0.05). Captopril also reduced the albumin excretion rate. However, the long term clinical benefit of reducing the progression from microalbuminuria to proteinuria has not been established.
Studies in rats and cats indicate that captopril does not cross the blood-brain barrier to any significant extent. |howSupplied=12.5 mg tablets in bottles of 100 (NDC 60505-0003-6) and 1000 (NDC 60505-0003-9), 25 mg tablets in bottles of 100 (NDC 60505-0004-6) and 1000 (NDC 60505-0004-9), 50 mg tablets in bottles of 100 (NDC 60505-0005-6) and 1000 (NDC 60505-0005-9), and 100 mg tablets in bottles of 100 (NDC 60505-0006-6) and 1000 (NDC 60505-0006-9).
The 12.5 mg tablet is a round, biconvex tablet, coded “APO 003” with a bisect score on one side and a plain face on the other; the 25 mg tablet is a round, biconvex tablet, coded “APO 004” on one side and a quadrisect score on the other; the 50 mg tablet is capsule shaped, biconvex tablet, coded “APO 005” with a bisect score on one side and a plain face on the other; the 100 mg tablet is capsule shaped, biconvex tablet, coded “APO 006” with a bisect score on one side and a plain face on the other.
All captopril tablets are white to off-white and may exhibit a slight sulfurous odor. |storage=Do not store above 86°F. Keep bottles tightly closed (protect from moisture). |fdaPatientInfo=For patient information about captopril from NLM, click here. |alcohol=Alcohol-Benazepril interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. }} {{#subobject:
|Page Name=Captopril |Pill Name=WW 173- Captopril.PNG |Drug Name=Captopril 50 MG Oral Tablet |Pill Ingred=Captopril|+sep=; |Pill Imprint=WW;173 |Pill Dosage=50 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=8 |Pill Scoring=2 |Pill Image= |Drug Author=West-ward Pharmaceutical Corp |NDC=0615-4519
}}
{{#subobject:
|Page Name=Captopril |Pill Name=M c 1- Captopril 12.5 mg.PNG |Drug Name=Captopril 12.5 MG Oral Tablet |Pill Ingred=Benazepril|+sep=; |Pill Imprint=M;C1 |Pill Dosage=12.5 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=8 |Pill Scoring=2 |Pill Image= |Drug Author=Novartis Pharamceuticals Corporation |NDC=0615-4520
}}
{{#subobject:
|Page Name=Captopril |Pill Name=W 7- Captopril 12.5 mg.PNG |Drug Name=Captopril 12.5 MG Oral Tablet |Pill Ingred=Benazepril|+sep=; |Pill Imprint=W;7 |Pill Dosage=12.5 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=8 |Pill Scoring=2 |Pill Image= |Drug Author=Novartis Pharmaceuticals Corporation |NDC=0615-4521
}}
{{#subobject:
|Label Page=Captopril |Label Name=Captopril_label_01.jpg
}}
{{#subobject:
|Label Page=Captopril |Label Name=Captopril_label_02.jpg
}}
{{#subobject:
|Label Page=Captopril |Label Name=Captopril_label_03.jpg
}}
{{#subobject:
|Label Page=Captopril |Label Name=Captopril_label_04.jpg
}}
{{#subobject:
|Label Page=Captopril |Label Name=Captopril_panel_01.jpg
}}
{{#subobject:
|Label Page=Captopril |Label Name=Captopril_panel_02.jpg
}}
{{#subobject:
|Label Page=Captopril |Label Name=Captopril_panel_03.jpg
}}
{{#subobject:
|Label Page=Captopril |Label Name=Captopril_panel_04.jpg
}}
- ↑ O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA et al. (2013) 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 127 (4):e362-425. DOI:10.1161/CIR.0b013e3182742cf6 PMID: 23247304
- ↑ Sloand JA, Izzo JL (1987) Captopril reduces urinary cystine excretion in cystinuria. Arch Intern Med 147 (8):1409-12. PMID: 2820331
- ↑ Perazella MA, Buller GK (1993) Successful treatment of cystinuria with captopril. Am J Kidney Dis 21 (5):504-7. PMID: 8488818
- ↑ Wang N, Zheng Z, Jin HY, Xu X (2012) Treatment effects of captopril on non-proliferative diabetic retinopathy. Chin Med J (Engl) 125 (2):287-92. PMID: 22340561
- ↑ Pisani F, Tisone G, Alciati E, Vennarecci G, Pieragostini E, Casciani CU (1994) Role of ACE inhibitors in the treatment of erythrocytosis in patients with renal allograft. Transplant Proc 26 (5):2602-3. PMID: 7940809
- ↑ Zucchelli P, Zuccalà A, Borghi M, Fusaroli M, Sasdelli M, Stallone C et al. (1992) Long-term comparison between captopril and nifedipine in the progression of renal insufficiency. Kidney Int 42 (2):452-8. PMID: 1405330
- ↑ Foucan L, Bourhis V, Bangou J, Mérault L, Etienne-Julan M, Salmi RL (1998) A randomized trial of captopril for microalbuminuria in normotensive adults with sickle cell anemia. Am J Med 104 (4):339-42. PMID: 9576406
- ↑ Kimmel PL, Mishkin GJ, Umana WO (1996) Captopril and renal survival in patients with human immunodeficiency virus nephropathy. Am J Kidney Dis 28 (2):202-8. PMID: 8768914
- ↑ González-Gay MA, García-Porrúa C, López Lázaro L, Olivé A (1998) Successful response to captopril in severe nephrotic syndrome secondary to lupus nephritis. Nephron 80 (3):353-4. PMID: 9807048
- ↑ Thibonnier M, Plouin PF, Ménard J, Corvol P (1983) [Primary hyperaldosteronism: diagnostic value of the administration of a single dose of captopril.] Ann Med Interne (Paris) 134 (3):251-5. PMID: 6354035
- ↑ Hené RJ, Koomans HA, Boer P, Dorhout Mees EJ (1982) Long-term treatment of bartter's syndrome with captopril. Br Med J (Clin Res Ed) 285 (6343):695. PMID: 6809195
- ↑ Aurell M, Rudin A (1983) Effect of captopril on blood pressure, renal function, the electrolyte balance and the renin-angiotensin system in Bartter's syndrome. Nephron 33 (4):274-8. PMID: 6341865
- ↑ Montigny M, Davignon A, Fouron JC, Biron P, Fournier A, Elie R (1989). "Captopril in infants for congestive heart failure secondary to a large ventricular left-to-right shunt". Am J Cardiol. 63 (9): 631–3. PMID 2645762.
- ↑ Chandar JJ, Sfakianakis GN, Zilleruelo GE, Guerra JJ, Georgiou MF, Abitbol CL; et al. (1999). "ACE inhibition scintigraphy in the management of hypertension in children". Pediatr Nephrol. 13 (6): 493–500. doi:10.1007/s004670050645. PMID 10452277.