Telbivudine adverse reactions
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Adverse Reactions
The following adverse reactions are discussed in other sections of the labeling:
- Lactic acidosis and severe hepatomegaly with steatosis [see Boxed Warning, Warnings and Precautions (5.1)]
- Severe acute exacerbations of hepatitis after discontinuation of treatment [see Boxed Warning, Warnings and Precautions (5.2)]
- Myopathy [see Warnings and Precautions (5.3)]
- Peripheral Neuropathy [see Warnings and Precautions (5.4)]
Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Assessment of adverse reactions is primarily based on two trials (007 GLOBE and NV-02B-015) in which 1,699 subjects with chronic hepatitis B received double-blind treatment with Tyzeka 600 mg per day (n=847 subjects) or lamivudine (n=852 subjects) for 104 weeks. The median duration of therapy was 104 weeks for both treatment groups.
In the 104 week clinical trials, most adverse experiences reported with Tyzeka were classified as mild or moderate in severity and were not attributed to Tyzeka. Selected adverse events of any severity which were reported in greater than or equal to 3% of Tyzeka and lamivudine recipients are shown in Table 2. With the exception of increased creatine kinase (CK), which was reported more frequently among Tyzeka recipients, the adverse event profile was similar for the two drugs.
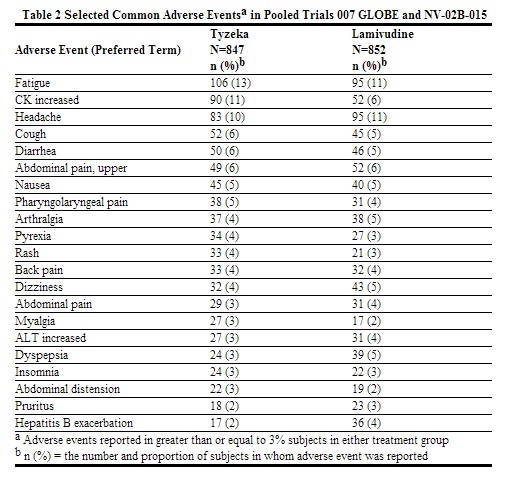 |
Moderate to severe (Grade 2-4) adverse events were reported in 239/847 (28%) of Tyzeka recipients and 229/852 (27%) of lamivudine recipients. The profile of adverse events of moderate to severe intensity was similar in both treatment groups and no individual adverse event was reported in greater than 2% of subjects in either treatment group.
Discontinuations due to adverse events were reported in 4% of Tyzeka recipients and 4% of lamivudine recipients. The most common adverse events resulting in Tyzeka discontinuation included increased CK, nausea, diarrhea, fatigue, myalgia, and myopathy.
Peripheral neuropathy was reported as an adverse event in less than 1% (2/847) of subjects receiving Tyzeka monotherapy [see Warnings and Precautions (5.4)]. Of Tyzeka-treated subjects less than 1% (5/847) were diagnosed with myopathy/myositis (presenting with muscular weakness) [see Warnings and Precautions (5.3)].
Laboratory Abnormalities
Frequencies of selected treatment-emergent laboratory abnormalities in the 007 GLOBE and NV-02B-015 trials are listed in Table 3.
 |
Creatine Kinase (CK) Elevations
Creatine kinase (CK) elevations were more frequent among subjects on Tyzeka treatment. By 104 weeks of treatment, Grade 1-4 CK elevations occurred in 79% of Tyzeka-treated subjects and 47% of lamivudine-treated subjects. Grade 3 or 4 CK elevations occurred in 13% of Tyzeka-treated subjects and 4% of lamivudine-treated subjects. Most CK elevations were asymptomatic, but the mean recovery time was longer for subjects on Tyzeka than subjects on lamivudine.
Among Tyzeka-treated subjects with Grade 1-4 CK elevations, 10% developed a musculoskeletal adverse event compared to 5% of lamivudine-treated subjects. A total of 2% (13/847) Tyzeka-treated subjects interrupted or discontinued trial drug due to CK elevation or musculoskeletal adverse events1.
______________________
1 Includes the Preferred Terms: back pain, chest wall pain, non-cardiac chest pain, chest discomfort, flank pain, muscle cramp, muscular weakness, musculoskeletal pain, musculoskeletal chest pain, musculoskeletal discomfort, musculoskeletal stiffness, myalgia, myofascial pain syndrome, myopathy, myositis, neck pain, and pain in extremity.
______________________
ALT Flares During Treatment
The incidence of ALT flares, defined as ALT greater than 10 x ULN and greater than 2 x baseline, was similar in the two treatment arms (3%) in the first six months. After week 24, ALT flares were reported less frequently in the Tyzeka arm (2%) compared to the lamivudine arm (5%). Periodic monitoring of hepatic function is recommended during chronic hepatitis B treatment.
Exacerbations of hepatitis after Discontinuation of Treatment
In the subset of subjects who discontinued treatment prematurely for reasons other than efficacy, or who elected not to continue Tyzeka in another clinical trial, 9/154 (6%) Tyzeka-treated and 10/180 (6%) lamivudine-treated subjects experienced an exacerbation of hepatitis (ALT elevation greater than 2 x baseline and greater than 10 x ULN) in the 4-month post-treatment period.
Results at 208 Weeks
After 104 weeks of blinded therapy in trials 007 GLOBE and NV-02B-015, 667 subjects received Tyzeka in an open-label extension trial, CLDT600A2303. Of those initially randomized to Tyzeka therapy, 78% of subjects (530/680) from trial 007 GLOBE and 82% (137/167) of subjects from trial NV-02B-015 enrolled into the extension trial and continued Tyzeka treatment for up to 208 weeks. The long-term Tyzeka safety population in trial CLDT600A2303 consisted of 655 subjects, including 518 subjects from trial 007 GLOBE and 137 subjects from trial NV-02B-015.
The overall safety profile from the pooled analysis up to 104 and 208 weeks was similar. Grade 3/4 CK elevations occurred in 16% of subjects (104/655) treated with Tyzeka in trial CLDT600A2303. Most grade 3/4 CK elevations were asymptomatic (74% of subjects without any muscle related adverse reaction) and transient (98% of episodes lasted one or two visits (visit interval 2 - 12 weeks) and 87% of subjects had one or two episodes). Most grade 3/4 CK elevations (93%) resolved spontaneously or returned to baseline levels. Two cases of myopathy and two cases of myositis were reported in the 655 Tyzeka-treated subjects.
Among the cohort of 655 subjects continuing Tyzeka for up to 208 weeks in trial CLDT600A2303, including the subgroup of patients (n=223) with mild renal impairment (eGFR 60-90 mL per min) at baseline, mean estimated GFR assessed by MDRD did not decline.
Postmarketing Experience
The following adverse reactions have been reported during post approval use of Tyzeka. Because these reactions were reported voluntarily from a population of unknown size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Musculoskeletal and Connective Tissue Disorders
Metabolism and Nutrition Disorders
Lactic acidosis[1]
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022011s013lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.