Telbivudine dosage and administration
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Dosage and Administration
Adults and Adolescents (16 years of age and older)
Due to higher rates of resistance that may develop with longer term treatment among patients with incomplete viral suppression, treatment should only be initiated, if pre-treatment HBV DNA and ALT measurements are known, in the following patient populations:
For HBeAg-positive patients, HBV DNA should be less than 9 log10 copies per mL and ALT should be greater than or equal to 2x ULN prior to treatment with Tyzeka.
For HBeAg-negative patients, HBV DNA should be less than 7 log10 copies per mL prior to treatment with Tyzeka.
HBV DNA levels should be monitored at 24 weeks of treatment to assure complete viral suppression (HBV DNA less than 300 copies per mL). Alternate therapy should be initiated for patients who have detectable HBV DNA after 24 weeks of treatment. Optimal therapy should be guided by further resistance testing.
The recommended dose of Tyzeka for the treatment of chronic hepatitis B is 600 mg once daily, taken orally, with or without food.
Tyzeka oral solution (30 mL) may be considered for patients who have difficulty with swallowing tablets.
Renal Impairment
Tyzeka may be used for the treatment of chronic hepatitis B in patients with impaired renal function. No adjustment to the recommended dose of Tyzeka is necessary in patients whose creatinine clearance is greater than or equal to 50 mL per min. Adjustment of the total daily dose of Tyzeka oral solution or of the interval for administration of Tyzeka tablets is required in patients with creatinine clearance less than 50 mL per min including those with ESRD on hemodialysis (Table 1).
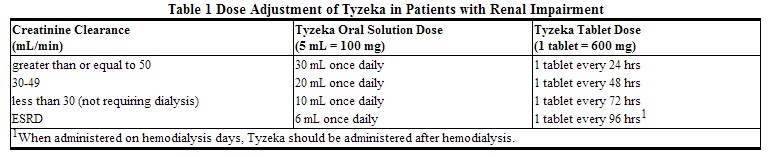 |
Hepatic Impairment
No adjustment to the recommended dose of Tyzeka is necessary in patients with hepatic impairment.
Duration of Therapy
For patients with incomplete viral suppression (HBV DNA greater than or equal to 300 copies per mL) after 24 weeks of treatment, alternate therapy should be instituted. HBV DNA should be monitored every 6 months to assure continued response. If patients test positive for HBV DNA at any time after their initial response, alternate treatment should be instituted. Optimal therapy should be guided by resistance testing.
The optimal duration of therapy with Tyzeka for patients with chronic hepatitis B is unknown.[1]
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022011s013lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.