Telbivudine microbiology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Microbiology
Antiviral Activity
The antiviral activity of telbivudine was assessed in the HBV-expressing human hepatoma cell line 2.2.15, as well as in primary duck hepatocytes infected with duck hepatitis B virus. The concentration of telbivudine that effectively inhibited 50% of viral DNA synthesis (EC50) in both systems was approximately 0.2 micromolar. The anti-HBV activity of telbivudine was additive with adefovir in cell culture, and was not antagonized by the HIV NRTIs didanosine and stavudine. Telbivudine was not antagonistic to the anti-HIV activity of abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir, or zidovudine. Transient reductions in HIV-1 RNA have been seen in some patients after administration of telbivudine in the absence of antiretroviral therapy. The clinical significance of these reductions has not been determined.
Resistance
Trial NV-02B-007 (007 GLOBE): In an as-treated analysis of the Phase III global registration trial, 59% (251/429) of treatment-naïve HBeAg-positive and 89% (202/227) of treatment-naïve HBeAg-negative subjects receiving Tyzeka 600 mg once daily achieved undetectable serum HBV DNA levels (less than 300 copies per mL) by Week 52. Of those who continued treatment beyond Week 52, 58% (243/418) and 85% (190/224) of HBeAg-positive and HBeAg-negative Tyzeka recipients, respectively, had undetectable HBV DNA at Week 104 (or at the end of dosing in treatment Year 2).
Genotypic analysis of paired baseline and treatment failure isolates from 181 evaluable subjects with amplifiable HBV DNA and greater than or equal to 16 weeks of Tyzeka treatment showed that the rtM204I/V substitution was associated with virologic failure (HBV DNA greater than or equal to 1,000 copies per mL) and virologic rebound (HBV DNA greater than or equal to 1 log10 increase above nadir). The rtM204I/V substitution was detectable in isolates from 78% (142/181) of evaluable subjects, and was frequently found with substitutions rtL80I/V and rtL180M. The rtM204I/V substitution was found infrequently with rtV27A, rtL82M, rtV173L, rtT184I/S, rtA200V, rtL229F/V/W, and rtR289K substitutions. The HBV of 16 subjects developed rtA181S/T amino acid substitutions while receiving Tyzeka. Eight of these 16 subjects had outgrowth of HBV expressing an rtM204I/V substitution without the rtA181 substitution and 1 subject’s HBV had both the rtM204I and rtA181T substitutions.
Trial CLDT600A2303: After 2 years of Tyzeka monotherapy in the 007 GLOBE trial, 77% (505/656) of subjects entered the open-label CLDT600A2303 extension trial to continue Tyzeka for up to 2 additional years, including 349 subjects who had undetectable levels of HBV DNA and 156 subjects who were viremic at entry. The rtM204I/V substitution was detectable in the virus from 83% (39/47) of the subjects losing viral suppression and having evaluable genotypic data. Of evaluable viremic subjects entering the extension, 25/33 (76%) developed rtM204I/V substitutions. Overall, 64 subjects developed genotypic resistance to Tyzeka with evidence of emerging rtM204I/V substitutions during the 2 years of Tyzeka treatment in this extension trial.
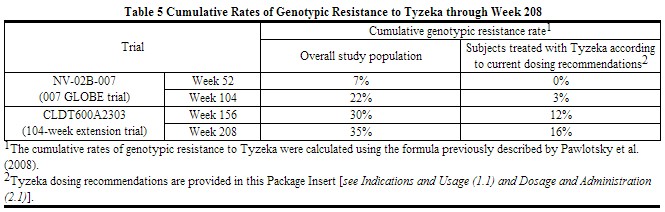
Subjects with higher baseline viral load had higher rates of genotypic resistance to Tyzeka, while subjects who achieved HBV DNA levels less than 300 copies per mL at Week 24 had lower rates of genotypic resistance to Tyzeka. The cumulative frequency of genotypic resistance (emergence of the rtM204I/V substitution) to Tyzeka in nucleos(t)ide treatment-naïve subjects was 7% and 22% at Weeks 52 and 104 of the controlled 007 GLOBE trial, and 30% and 35% at Weeks 156 and 208 of the open-label extension trial (CLDT600A2303), respectively (Table 5).
One-hundred-sixty-seven subjects (25% of those in the 007 GLOBE trial) were treated with Tyzeka according to current dosing recommendations [see Indications and Usage (1.1)]. Eighty-four percent (140/167) of these subjects qualified at 24 weeks for continued Tyzeka treatment (HBV DNA less than 300 copies per mL). Retrospective calculation of the cumulative rate of genotypic resistance to Tyzeka for this subgroup of subjects was 0%, 3%, 12%, and 16% at Weeks 52, 104, 156, and 208, respectively (Table 5).
 |
Cross-Resistance
Cross-resistance has been observed among HBV nucleos(t)ide analogues. In cell-based assays, lamivudine-resistant HBV strains expressing either the rtM204I substitution or the rtL180M/rtM204V double substitution had greater than or equal to 1,000-fold reduced susceptibility to telbivudine. Telbivudine retained wild-type phenotypic activity (1.2-fold reduction) against HBV expressing rtM204V alone. Most subjects (92%, 155/169) whose virus developed lamivudine resistance-associated substitutions (rtM204I/V) during 2 years of lamivudine treatment in the 007 GLOBE trial remained viremic (HBV DNA greater than 300 copies per mL) after up to 2 years of Tyzeka monotherapy in the CLDT600A2303 extension trial, including 91% (50/55) of the subjects with the rtM204V substitution.
HBV encoding the adefovir resistance-associated substitution rtA181V showed 3- to 5-fold reduced susceptibility to telbivudine in cell culture. The rtA181S and rtA181T substitutions conferred 2.7- and 3.5-fold reductions in susceptibility to telbivudine, respectively. The rtA181T substitution is associated with decreased clinical response in subjects with HBV treated with adefovir and entecavir. HBV encoding the adefovir resistance-associated substitution rtN236T remained susceptible to telbivudine.[1]
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022011s013lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.