Saquinavir mesylate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Saquinavir mesylate is a protease inhibitor that is FDA approved for the treatment of HIV-1 infection in adults. Common adverse reactions include lipodystrophy, abdominal pain, diarrhea, nausea and vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Saquinavir mesylate in combination with ritonavir and other antiretroviral agents is indicated for the treatment of HIV-1 infection in adults (over the age of 16 years).
- Dosage: Saquinavir mesylate 1000 mg twice daily (5 × 200-mg capsules or 2 × 500-mg tablets) in combination with ritonavir 100-mg twice daily.
- Ritonavir should be taken at the same time as saquinavir mesylate.
- Saquinavir mesylate and ritonavir should be taken within 2 hours after a meal.
- No additional ritonavir is recommended when saquinavir mesylate is administered with lopinavir/ritonavir 400/100 mg twice daily.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Saquinavir mesylate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Saquinavir mesylate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Saquinavir mesylate in combination with ritonavir and other antiretroviral agents is indicated for the treatment of HIV-1 infection in patients over the age of 16 years.
- Dosage: Saquinavir mesylate 1000 mg twice daily (5 × 200-mg capsules or 2 × 500-mg tablets) in combination with ritonavir 100-mg twice daily.
- Ritonavir should be taken at the same time as saquinavir mesylate.
- Saquinavir mesylate and ritonavir should be taken within 2 hours after a meal.
- No additional ritonavir is recommended when saquinavir mesylate is administered with lopinavir/ritonavir 400/100 mg twice daily.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Saquinavir mesylate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Saquinavir mesylate in pediatric patients.
Contraindications
QT interval prolongation and torsades de pointes have been reported rarely with saquinavir mesylate/ritonavir use. Do not use in patients with congenital long QT syndrome, those with refractory hypokalemia or hypomagnesemia, and in combination with drugs that both increase saquinavir plasma concentrations and prolong the QT interval.
Saquinavir mesylate is contraindicated in:
- Patients with complete atrioventricular (AV) block without implanted pacemakers, or patients who are at high risk of complete AV block.
- Patients with clinically significant hypersensitivity (e.g., anaphylactic reaction, Stevens-Johnson syndrome) to saquinavir, saquinavir mesylate, or any of its ingredients.
- Patients with severe hepatic impairment when is administered with ritonavir.
Coadministration of saquinavir mesylate/ritonavir is contraindicated with drugs that are CYP3A substrates for which increased plasma levels may result in serious or life-threatening reactions. These drugs and potentially related adverse events are listed in TABLE 1.
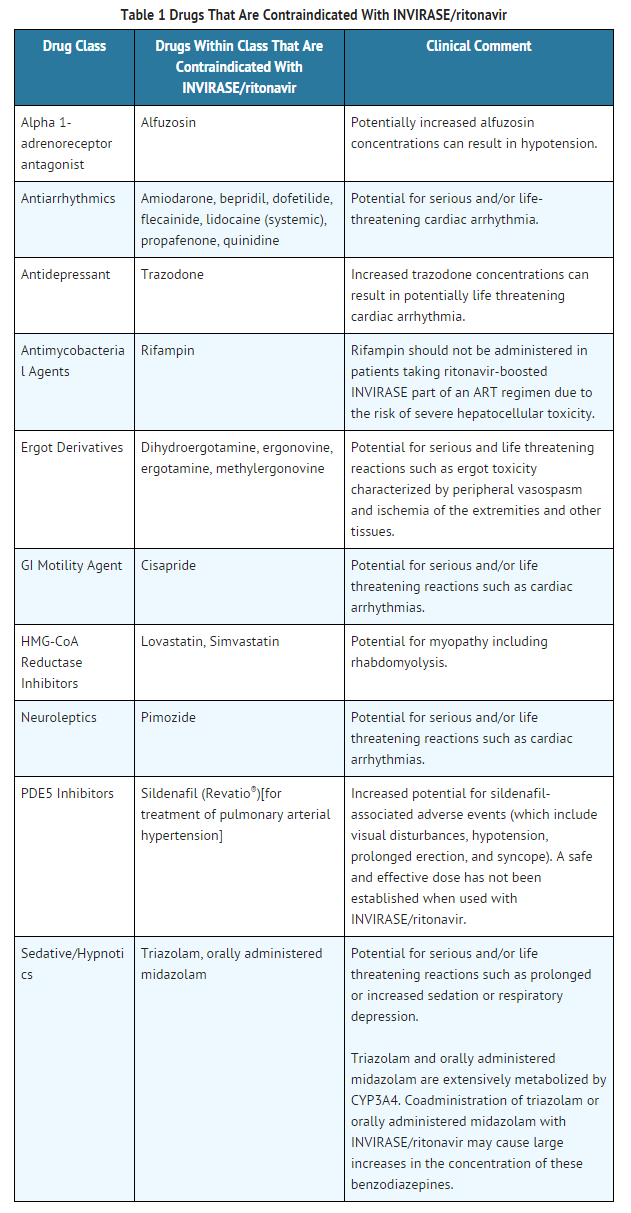
Warnings
Saquinavir mesylate must be used in combination with ritonavir. Please refer to the ritonavir full prescribing information for additional precautionary measures.
If a serious or severe toxicity occurs during treatment with saquinavir mesylate, saquinavir mesylate should be interrupted until the etiology of the event is identified or the toxicity resolves. At that time, resumption of treatment with full-dose saquinavir mesylate may be considered. For antiretroviral agents used in combination with saquinavir mesylate, physicians should refer to the complete product information for these drugs for dose adjustment recommendations and for information regarding drug-associated adverse reactions.
The combination saquinavir mesylate/ritonavir is a potent inhibitor of CYP3A and may significantly increase the exposure of drugs primarily metabolized by CYP3A.
Saquinavir/ritonavir prolongs the PR interval in a dose-dependent fashion. Cases of second or third degree atrioventricular block have been reported rarely. Patients with underlying structural heart disease, pre-existing conduction system abnormalities, cardiomyopathies and ischemic heart disease may be at increased risk for developing cardiac conduction abnormalities. ECG monitoring is recommended in these patients.
The impact on the PR interval of co-administration of saquinavir/ritonavir with other drugs that prolong the PR interval (including calcium channel blockers, beta-adrenergic blockers, digoxin and atazanavir) has not been evaluated. As a result, co-administration of saquinavir/ritonavir with these drugs should be undertaken with caution, particularly with those drugs metabolized by CYP3A, and clinical monitoring is recommended.
Saquinavir/ritonavir causes dose-dependent QT prolongation. Torsades de pointes has been reported rarely post-marketing. Avoid saquinavir/ritonavir in patients with long QT syndrome. ECG monitoring is recommended if therapy is initiated in patients with congestive heart failure, bradyarrhythmias, hepatic impairment and electrolyte abnormalities. Correct hypokalemia or hypomagnesemia prior to initiating saquinavir/ritonavir and monitor these electrolytes periodically during therapy. Do not use in combination with drugs that both increase saquinavir plasma concentrations and prolong the QT interval.
Patients initiating therapy with ritonavir-boosted saquinavir mesylate:
- An ECG should be performed prior to initiation of treatment. Patients with a QT interval > 450 msec should not receive ritonavir-boosted saquinavir mesylate. For patients with a QT interval < 450 msec, an on-treatment ECG is suggested after approximately 3 to 4 days of therapy; patients with a QT interval > 480 msec or prolongation over pre-treatment by > 20 msec should discontinue ritonavir-boosted saquinavir mesylate.
Patients requiring treatment with medications with the potential to increase the QT interval and concomitant ritonavir-boosted saquinavir mesylate:
- Such combinations should only be used where no alternative therapy is available and the potential benefits outweigh the potential risks. An ECG should be performed prior to initiation of the concomitant therapy, and patients with a QT interval > 450 msec should not initiate the concomitant therapy. If baseline QT interval < 450 msec, an on-treatment ECG should be performed after 3-4 days of therapy. For patients demonstrating a subsequent increase in QT interval to > 480 msec or increase by > 20 msec after commencing concomitant therapy, the physician should use best clinical judgment to discontinue either ritonavir-boosted saquinavir mesylate or the concomitant therapy or both.
A cardiology consult is recommended if drug discontinuation or interruption is being considered on the basis of ECG assessment.
New onset diabetes mellitus, exacerbation of preexisting diabetes mellitus and hyperglycemia have been reported during postmarketing surveillance in HIV-1-infected patients receiving protease-inhibitor therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for the treatment of these events. In some cases diabetic ketoacidosis has occurred. In those patients who discontinued protease-inhibitor therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and a causal relationship between protease-inhibitor therapy and these events has not been established.
In patients with underlying hepatitis B or C, cirrhosis, chronic alcoholism and/or other underlying liver abnormalities, there have been reports of worsening liver disease.
There have been reports of spontaneous bleeding in patients with hemophilia A and B treated with protease inhibitors. In some patients additional factor VIII was required. In the majority of reported cases treatment with protease inhibitors was continued or restarted. A causal relationship between protease inhibitor therapy and these episodes has not been established.
Elevated cholesterol and/or triglyceride levels have been observed in some patients taking saquinavir in combination with ritonavir. Marked elevation in triglyceride levels is a risk factor for development of pancreatitis. Cholesterol and triglyceride levels should be monitored prior to initiating combination dosing regimen of saquinavir mesylate with ritonavir, and at periodic intervals while on such therapy. In these patients, lipid disorders should be managed as clinically appropriate.
Each capsule contains lactose (anhydrous) 63.3 mg. This quantity should not induce specific symptoms of intolerance.
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), facial wasting, peripheral wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including saquinavir mesylate. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jiroveci pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
Varying degrees of cross-resistance among HIV-1 protease inhibitors have been observed. Continued administration of saquinavir mesylate therapy following loss of viral suppression may increase the likelihood of cross resistance to other protease inhibitors.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The original saquinavir mesylate safety database consisted of a total of 574 adult subjects who received saquinavir 600 mg alone or in combination with ZDV or ddC. Combination dosing with ritonavir is based on 352 HIV-1 infected subjects and 166 healthy subjects who received various combinations of either saquinavir (hard gel or soft-gel capsules) with ritonavir.
The recommended dose of saquinavir mesylate is 1000 mg twice daily co-administered with ritonavir 100 mg twice daily, in combination with other antiretroviral agents. TABLE 2 lists grade 2, 3 and 4 adverse events that occurred in ≥2% of subjects receiving saquinavir soft gel capsules with ritonavir (1000/100 mg bid).

Limited experience is available from three trials investigating the pharmacokinetics of the saquinavir mesylate 500 mg film-coated tablet compared to the saquinavir mesylate 200 mg capsule in healthy volunteers (n=140). In two of these trials saquinavir was boosted with ritonavir; in the other trial, saquinavir was administered as single drug. The saquinavir mesylate tablet and the capsule formulations were similarly tolerated. The most common adverse events were gastrointestinal disorders (such as nausea, vomiting, and diarrhea). Similar bioavailability was demonstrated and no clinically significant differences in saquinavir exposures were seen. Thus, similar safety profiles are expected between the two saquinavir mesylate formulations.
A study investigating the drug-drug interaction of rifampin 600 mg/day daily and saquinavir mesylate 1000 mg/ritonavir 100 mg twice daily enrolled 28 healthy volunteers. Eleven of 17 healthy volunteers (65%) exposed concomitantly to rifampin and ritonavir-boosted saquinavir mesylate developed severe hepatocellular toxicity which presented as increased hepatic transaminases. In some subjects, transaminases increased up to >20-fold the upper limit of normal and were associated with gastrointestinal symptoms, including abdominal pain, gastritis, nausea, and vomiting. Following discontinuation of all three drugs, clinical symptoms abated and the increased hepatic transaminases normalized.
Additional Adverse Reactions Reported During Clinical Trials with Saquinavir
- Blood and lymphatic system disorders: anemia, hemolytic anemia, leukopenia, lymphadenopathy, neutropenia, pancytopenia, thrombocytopenia
- Cardiac disorders: heart murmur, syncope
- Ear and labyrinth disorders: tinnitus
- Eye disorders: visual impairment
- Gastrointestinal disorders: abdominal discomfort, ascites, dyspepsia, dysphagia, eructation, flatulence, gastritis, gastrointestinal hemorrhage, intestinal obstruction, mouth dry, mucosal ulceration, pancreatitis
- General disorders and administration site conditions: anorexia, asthenia, chest pain, edema, lethargy, wasting syndrome, weight increased
- Hepatobiliary disorders: chronic active hepatitis, hepatitis, hepatomegaly, hyperbilirubinemia, jaundice, portal hypertension
- Immune system disorders: allergic reaction
- Investigations: ALT increase, AST increase, blood creatine phosphokinase increased, increased alkaline phosphatase, GGT increase, raised amylase, raised LDH
- Metabolism and nutrition disorders: increased or decreased appetite, dehydration, hypertriglyceridemia
- Musculoskeletal and connective tissue disorders: arthralgia, muscle spasms, myalgia, polyarthritis
- Neoplasms benign, malignant and unspecified (incl cysts and polyps): acute myeloid leukemia, papillomatosis
- Nervous system disorders: confusion, convulsions, coordination abnormal, dizziness, dysgeusia, headache, hypoaesthesia, intracranial hemorrhage leading to death, loss of consciousness, paresthesia, peripheral neuropathy, somnolence, tremor
- Psychiatric disorders: anxiety, depression, insomnia, libido disorder, psychotic disorder, sleep disorder, suicide attempt
- Renal and urinary disorders: nephrolithiasis
- Respiratory, thoracic and mediastinal disorders: cough, dyspnea
- Skin and subcutaneous tissue disorders: acne, alopecia, dermatitis bullous, drug eruption, erythema, severe cutaneous reaction associated with increased liver function tests, Stevens-Johnson syndrome, sweating increased, urticaria
- Vascular disorders: hypertension, hypotension, thrombophlebitis, peripheral vasoconstriction
Limited safety data are available from two pediatric clinical trials of saquinavir hard gel capsules (approximately 50 mg per kg twice daily) used in combination with either low dose ritonavir or lopinavir/ritonavir. These trials enrolled pediatric subjects aged 4 months to 16 years old. In the HIVNAT 017 study (saquinavir mesylate + lopinavir/ritonavir), adverse events were reported in 90% of the 50 subjects enrolled. The most commonly reported adverse events considered related to study treatment were diarrhea (18%) and vomiting (10%). In the NV20911 study (saquinavir mesylate + ritonavir), 4 subjects (22% of 18 enrolled) experienced adverse events that were considered related to saquinavir mesylate + ritonavir. These events were vomiting, abdominal pain and diarrhea. All reported adverse events were mild or moderate in intensity. The adverse reaction profile of saquinavir mesylate in the pediatric trials is similar to that observed in adult trials.
Postmarketing Experience
Additional adverse events identified during postmarketing use are similar to those observed in clinical trials with saquinavir mesylate and saquinavir soft gel capsules alone or in combination with ritonavir. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to saquinavir mesylate exposure. In addition, torsades de pointes has been reported rarely.
Drug Interactions
Drug interaction studies have been completed with both saquinavir mesylate and saquinavir soft gel capsules. Observations from drug interaction studies with saquinavir soft gel capsules may not be predictive for saquinavir mesylate/ritonavir. Because ritonavir is coadministered with saquinavir mesylate, prescribers should also refer to the prescribing information for ritonavir regarding drug interactions associated with this agent.
The combination saquinavir mesylate/ritonavir is a potent inhibitor of CYP3A and may significantly increase the exposure of drugs primarily metabolized by CYP3A. Drugs that are contraindicated specifically due to the observed or expected magnitude of interaction and potential for serious or life-threatening adverse events are listed in TABLE 1. Coadministration with other CYP3A substrates may require a dose adjustment or additional monitoring.
The metabolism of saquinavir is mediated primarily by CYP3A. Additionally, saquinavir is a substrate for P-glycoprotein (P-gp). Therefore, drugs that affect CYP3A and/or P-gp may modify the pharmacokinetics of saquinavir. Coadministration with drugs that are potent inducers of CYP3A (e.g., phenobarbital, phenytoin, carbamazepine) may result in decreased plasma concentrations of saquinavir and reduced therapeutic effect.
Based on the finding of dose-dependent prolongations of QT and PR intervals in healthy volunteers receiving saquinavir mesylate/ritonavir, additive effects on QT and/or PR interval prolongation may occur with certain members of the following drug classes: antiarrhythmics class IA or class III, neuroleptics, antidepressive agents, PDE5 inhibitors (when used for pulmonary arterial hypertension), antimicrobials, antihistaminics and others. This effect might lead to an increased risk of ventricular arrhythmias, notably torsades de pointes. Therefore, concurrent administration of these agents with saquinavir mesylate/ritonavir is contraindicated.

Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
Reproduction studies conducted with saquinavir have shown no embryotoxicity or teratogenicity in both rats and rabbits. Because of limited bioavailability of saquinavir in animals and/or dosing limitations, the plasma exposures (AUC values) in the respective species were approximately 29% (using rat) and 21% (using rabbit) of those obtained in humans at the recommended clinical dose boosted with ritonavir. Clinical experience in pregnant women is limited. Saquinavir should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS): B1
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Saquinavir mesylate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Saquinavir mesylate during labor and delivery.
Nursing Mothers
Do not breastfeed if HIV-1-infected mothers are receiving saquinavir mesylate therapy.
Pediatric Use
Steady state saquinavir exposures observed in pediatric trials were substantially higher than historical data in adults where dose- and exposure-dependent QTc and PR prolongation were observed. Although electrocardiogram abnormalities were not reported in these pediatric trials, the trials were small and not designed to evaluate QT or PR intervals. Modeling and simulation assessment of pharmacokinetic/pharmacodynamic relationships in pediatric subjects suggest that reducing the saquinavir mesylate dose to minimize risk of QT prolongation is likely to reduce antiviral efficacy. In addition, no clinical efficacy data are available at saquinavir mesylate doses less than 50 mg per kg in pediatric subjects. Therefore, pediatric dose recommendations that are both reliably effective and below thresholds of concern with respect to QT and PR prolongation could not be determined.
Geriatic Use
Clinical trials of saquinavir mesylate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dosing saquinavir mesylate in elderly patients should be undertaken with caution keeping in mind the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
A gender difference was observed, with females showing higher saquinavir exposure than males (mean AUC 56% higher, mean Cmax 26% higher), in the relative bioavailability study comparing saquinavir mesylate 500 mg film-coated tablets to the saquinavir mesylate 200 mg capsules in combination with ritonavir. There was no evidence that age and body weight explained the gender difference in this study. A clinically significant difference in safety and efficacy between men and women has not been reported with the approved dosage regimen (saquinavir 1000-mg/ritonavir 100-mg twice daily).
Race
The effect of race on the pharmacokinetics of saquinavir has not been investigated.
Renal Impairment
Renal clearance is a minor elimination pathway; the principal route of excretion for saquinavir is by hepatic metabolism. Therefore, no initial dose adjustment is necessary for patients with renal impairment. However, patients with severe renal impairment or end-stage renal disease (ESRD) have not been studied, and caution should be exercised when prescribing saquinavir mesylate in this population.
Hepatic Impairment
No dosage adjustment is necessary for HIV-1-infected patients with mild or moderate hepatic impairment based on limited data. In patients with underlying hepatitis B or C, cirrhosis, chronic alcoholism and/or other underlying liver abnormalities, there have been reports of worsening liver disease. saquinavir mesylate when administered with ritonavir is contraindicated in patients with severe hepatic impairment.
Females of Reproductive Potential and Males
No adverse effects were reported in fertility and reproductive performance study conducted in rats. Because of limited bioavailability of saquinavir in animals, the maximal plasma exposures achieved in rats were approximately 26% of those obtained in humans at the recommended clinical dose boosted with ritonavir.
Immunocompromised Patients
There is no FDA guidance one the use of Saquinavir mesylate in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
There is limited information regarding Saquinavir mesylate Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Saquinavir mesylate and IV administrations.
Overdosage
- There is limited experience of overdose with saquinavir.
- No acute toxicities or sequelae were noted in 1 subject who ingested 8 grams of saquinavir mesylate as a single dose. The subject was treated with induction of emesis within 2 to 4 hours after ingestion. A second subject ingested 2.4 grams of saquinavir mesylate in combination with 600 mg of ritonavir and experienced pain in the throat that lasted for 6 hours and then resolved. In an exploratory Phase II study of oral dosing with saquinavir mesylate at 7200 mg per day (1200 mg q4h), there were no serious toxicities reported through the first 25 weeks of treatment.
- Treatment of overdose with saquinavir should consist of general supportive measures including monitoring of vital signs and ECG and observations of the patient's clinical status. Since saquinavir is highly protein bound, dialysis is unlikely to be beneficial in significant removal of the active substance.
Pharmacology
| |
Saquinavir mesylate
| |
| Systematic (IUPAC) name | |
| (2S)-N-[(2S,3R)-4-[(3S)-3-(tert-butylcarbamoyl)-decahydroisoquinolin-2-yl]-3-hydroxy-1-phenylbutan-2-yl]-2-(quinolin-2-ylformamido)butanediamide | |
| Identifiers | |
| CAS number | |
| ATC code | J05 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 670.841 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 98% |
| Metabolism | ? |
| Half life | 9 - 15 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
B1 (Australia) |
| Legal status | |
| Routes | ? |
Mechanism of Action
Saquinavir is an inhibitor of HIV-1 protease. HIV-1 protease is an enzyme required for the proteolytic cleavage of viral polyprotein precursors into individual functional proteins found in HIV-1 particles. Saquinavir is a peptide-like substrate analogue that binds to the protease active site and inhibits the activity of the enzyme. Saquinavir inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature noninfectious viral particles.
Structure
Saquinavir mesylate has the following structural formula:

Pharmacodynamics
QTcS interval was evaluated in a randomized, placebo and active (moxifloxacin 400 mg once daily) controlled crossover study in 59 healthy adults, with ECG measurements on Day 3. The maximum mean (95% upper confidence bound) differences in QTcS interval from placebo after baseline-correction were 18.9 (22.0) and 30.2 (33.4) ms for 1000/100 mg twice daily and supratherapeutic 1500/100 mg twice daily of saquinavir mesylate/ritonavir, respectively. There is a delayed effect between QTc interval change and drug concentrations, with the maximum placebo-adjusted baseline-corrected QTcS observed at about 12-20 h post-dose. saquinavir mesylate/ritonavir 1500/100 mg twice daily resulted in a Day 3 mean Cmax of saquinavir mesylate approximately 1.4-fold higher than the mean Cmax observed on Day 3 with the approved therapeutic dose in healthy volunteers (within the same study). QTcS in this study was QT/RR0.319 for males and QT/RR0.337 for females, which are similar to Fridericia's correction (QTcF=QT/RR0.3333).
PR and QRS interval prolongations were also noted in subjects receiving saquinavir mesylate/ritonavir in the same study on Day 3. The maximum mean (95% upper confidence bound) difference from placebo in the PR interval after baseline-correction were 28.6 (31.6) and 38.4 (41.4) ms for 1000/100 mg twice daily and supratherapeutic 1500/100 mg twice daily saquinavir/ritonavir respectively. The maximum mean (95% upper confidence bound) difference from placebo in QRS interval after baseline correction were 2.9 (3.9) and 4.4 (5.3) ms for 1000/100 mg twice daily and supratherapeutic 1500/100 mg twice daily saquinavir mesylate/ritonavir respectively. In this study using healthy subjects, PR interval prolongation of >200 ms was also observed in 40% and 47% of subjects receiving saquinavir mesylate/ritonavir 1000/100 mg bid and 1500/100 mg bid, respectively, on Day 3. Three (3%) of subjects in the active control moxifloxacin arm and 5% in the placebo arm experienced PR prolongation of >200 ms.
Pharmacokinetics
The pharmacokinetics of saquinavir mesylate/ritonavir 1000/100 mg twice daily have been evaluated in HIV-1-infected subjects and healthy subjects. Steady-state saquinavir AUC, Cmax, and Cmin in healthy subjects are approximately 50% higher than observed in HIV-1-infected subjects.
Adults
Absorption and Bioavailability
Similar bioavailability was demonstrated when saquinavir mesylate 500 mg film-coated tablet (2 × 500 mg) and saquinavir mesylate 200 mg capsule (5 × 200 mg) were administered with low-dose ritonavir (100 mg) under fed conditions. The ratio of mean exposures (90% confidence intervals) of tablets vs capsules was 1.10 (1.04-1.16) for AUC0-∞ and 1.19 (1.14-1.25) for Cmax.
Absolute bioavailability of saquinavir administered as saquinavir mesylate averaged 4% (CV 73%, range: 1% to 9%) in 8 healthy volunteers who received a single 600-mg dose (3 × 200 mg) of saquinavir mesylate following a high-fat breakfast (48 g protein, 60 g carbohydrate, 57 g fat; 1006 kcal). The low bioavailability is thought to be due to a combination of incomplete absorption and extensive first-pass metabolism.
saquinavir mesylate in combination with ritonavir at a dose of 1000/100 mg twice daily provides saquinavir systemic exposures over a 24-hour period that are similar to those achieved with saquinavir soft gel capsules with ritonavir 1000/100 mg twice daily and greater than that achieved with saquinavir soft gel capsules 1200 mg three times daily.

Food Effect
The mean 24-hour AUC after a single 600-mg oral dose (6 × 100 mg) in healthy volunteers (n=6) was increased from 24 ng∙h/mL (CV 33%), under fasting conditions, to 161 ng∙h/mL (CV 35%) when saquinavir mesylate was given following a high-fat breakfast (48 g protein, 60 g carbohydrate, 57 g fat; 1006 kcal). Saquinavir 24-hour AUC and Cmax (n=6) following the administration of a higher calorie meal (943 kcal, 54 g fat) were on average 2 times higher than after a lower calorie, lower fat meal (355 kcal, 8 g fat). The effect of food has been shown to persist for up to 2 hours.
saquinavir mesylate/ritonavir should be taken within 2 hours after a meal.
Distribution
The mean steady-state volume of distribution following intravenous administration of a 12-mg dose of saquinavir (n=8) was 700 L (CV 39%), suggesting saquinavir partitions into tissues. Saquinavir was approximately 98% bound to plasma proteins over a concentration range of 15 to 700 ng/mL. In 2 subjects receiving saquinavir mesylate 600 mg three times daily, cerebrospinal fluid concentrations were negligible when compared to concentrations from matching plasma samples.
Metabolism and Elimination
In vitro studies using human liver microsomes have shown that the metabolism of saquinavir is cytochrome P450 mediated with the specific isoenzyme, CYP3A4, responsible for more than 90% of the hepatic metabolism. Based on in vitro studies, saquinavir is rapidly metabolized to a range of mono- and di-hydroxylated inactive compounds. In a mass balance study using 600 mg 14C-saquinavir mesylate (n=8), 88% and 1% of the orally administered radioactivity was recovered in feces and urine, respectively, within 5 days of dosing. In an additional 4 subjects administered 10.5 mg 14C-saquinavir intravenously, 81% and 3% of the intravenously administered radioactivity was recovered in feces and urine, respectively, within 5 days of dosing. In mass balance studies, 13% of circulating radioactivity in plasma was attributed to unchanged drug after oral administration and the remainder attributed to saquinavir metabolites. Following intravenous administration, 66% of circulating radioactivity was attributed to unchanged drug and the remainder attributed to saquinavir metabolites, suggesting that saquinavir undergoes extensive first-pass metabolism.
Systemic clearance of saquinavir was rapid, 1.14 L/h/kg (CV 12%) after intravenous doses of 6, 36, and 72 mg. The mean residence time of saquinavir was 7 hours (n=8).
Nonclinical Toxicology
Antiviral Activity
The antiviral activity of saquinavir was assessed in lymphoblastoid and monocytic cell lines and in peripheral blood lymphocytes in cell culture. Saquinavir inhibited HIV-1 activity in both acutely and chronically infected cells. EC50 and EC90 values (50% and 90% inhibitory concentrations) ranged from 1 to 30 nM and 5 to 80 nM, respectively. In the presence of 40% human serum, the mean EC50 of saquinavir against laboratory strain HIV-1 RF in MT4 cells was 37.7± 5 nM representing a 4-fold increase in the EC50 value. In cell culture, saquinavir demonstrated additive to synergistic effects against HIV-1 in combination with reverse transcriptase inhibitors (didanosine, lamivudine, nevirapine, stavudine and zidovudine) without enhanced cytotoxicity. Saquinavir in combination with the protease inhibitors amprenavir, atazanavir, or lopinavir resulted in synergistic antiviral activity. Saquinavir displayed antiviral activity in cell culture against HIV-1 clades A-H (EC50 values ranged from 0.9 to 2.5 nM). The EC50 and EC90 values of saquinavir against HIV-2 isolates in cell culture ranged from 0.25 nM to 14.6 nM and 4.65 nM to 28.6 nM, respectively.
Resistance
HIV-1 isolates with reduced susceptibility to saquinavir have been selected during passage in cell culture. Genotypic analyses of these isolates showed several amino acid substitutions in the HIV-1 protease. Only the G48V and L90M substitutions were associated with reduced susceptibility to saquinavir, and conferred an increase in the EC50 value of 8- and 3-fold, respectively.
HIV-1 isolates with reduced susceptibility (≥4-fold increase in the EC50 value) to saquinavir emerged in some subjects treated with saquinavir mesylate. Genotypic analysis of these isolates identified resistance conferring primary amino acid substitutions in the protease G48V and L90M, and secondary substitutions L10I/R/V, I54V/L, A71V/T, G73S, V77I, V82A and I84V that contributed additional resistance to saquinavir. Forty-one isolates from 37 subjects failing therapy with saquinavir mesylate had a median decrease in susceptibility to saquinavir of 4.3-fold.
The degree of reduction in cell culture susceptibility to saquinavir of clinical isolates bearing substitutions G48V and L90M depends on the number of secondary substitutions present. In general, higher levels of resistance are associated with greater number of substitutions only in association with either or both of the primary substitutions G48V and L90M. No data are currently available to address the development of resistance in patients receiving saquinavir/ritonavir.
Cross-resistance
Among protease inhibitors, variable cross-resistance has been observed. In one clinical study, 22 HIV-1 isolates with reduced susceptibility (>4-fold increase in the EC50 value) to saquinavir following therapy with saquinavir mesylate were evaluated for cross-resistance to amprenavir, indinavir, nelfinavir and ritonavir. Six of the 22 isolates (27%) remained susceptible to all 4 protease inhibitors, 12 of the 22 isolates (55%) retained susceptibility to at least one of the protease inhibitors and 4 out of the 22 isolates (18%) displayed broad cross-resistance to all protease inhibitors. Sixteen (73%) and 11 (50%) of the 22 isolates remained susceptible (<4-fold) to amprenavir and indinavir, respectively. Four of 16 (25%) and nine of 21 (43%) with available data remained susceptible to nelfinavir and ritonavir, respectively.
After treatment failure with amprenavir, cross-resistance to saquinavir was evaluated. HIV-1 isolates from 22/22 subjects failing treatment with amprenavir and containing one or more substitutions M46L/I, I50V, I54L, V32I, I47V, and I84V were susceptible to saquinavir.
Carcinogenesis
Carcinogenicity studies found no indication of carcinogenic activity in rats and mice administered saquinavir for approximately 2 years. Because of limited bioavailability of saquinavir in animals, the plasma exposures (AUC values) in the respective species were approximately 29% (using rat) and 65% (using mouse) of those obtained in humans at the recommended clinical dose boosted with ritonavir.
Mutagenesis
Mutagenicity and genotoxicity studies, with and without metabolic activation where appropriate, have shown that saquinavir has no mutagenic activity in vitro in either bacterial (Ames test) or mammalian cells (Chinese hamster lung V79/HPRT test). Saquinavir does not induce chromosomal damage in vivo in the mouse micronucleus assay or in vitro in human peripheral blood lymphocytes, and does not induce primary DNA damage in vitro in the unscheduled DNA synthesis test.
Clinical Studies
In a randomized, double-blind clinical study NV14256 in zidovudine-experienced, HIV-1-infected adult subjects, saquinavir mesylate in combination with zalcitabine1 was shown to be superior to either saquinavir mesylate or zalcitabine monotherapy in decreasing the cumulative incidence of clinical disease progression to AIDS-defining events or death. In another randomized study ACTG229/NV14255, subjects with advanced HIV-1 infection with history of prolonged zidovudine treatment were administered saquinavir mesylate 600 mg (three times daily) + zidovudine + zalcitabine. Subjects receiving this regimen experienced greater increases in CD4+ cell counts as compared to those who received saquinavir mesylate + zidovudine or zalcitabine + zidovudine. It should be noted the HIV treatment regimens that were used in these clinical trials are no longer considered standard of care.
In the MaxCmin1 trial, saquinavir gel capsule 1000 mg twice daily coadministered with ritonavir 100 mg twice daily was evaluated in a heterogeneous population of 148 HIV-1-infected subjects. A total of 42 subjects enrolled were treatment naïve, and 106 subjects were treatment experienced (of which 52 subjects had HIV-1 RNA < 400 copies/mL at baseline). Results showed that 91/148 (61%) subjects achieved and/or sustained an HIV-1 RNA <400 copies per mL at the completion of 48 weeks treatment.
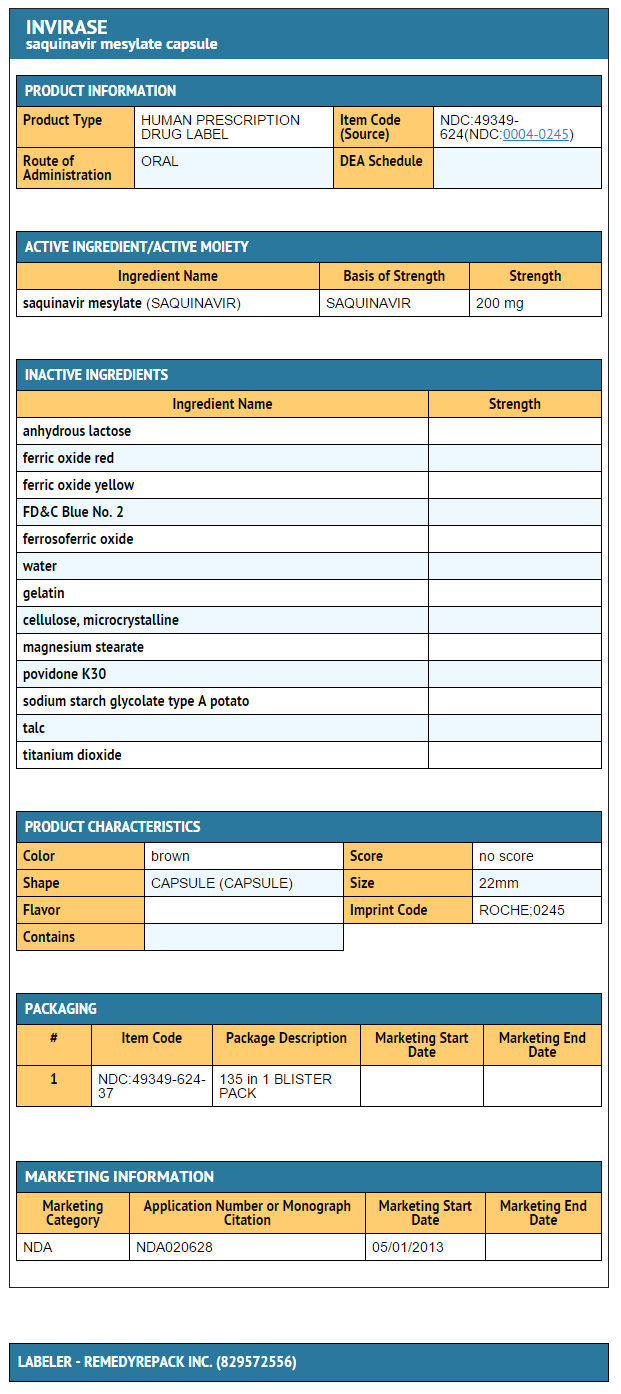
How Supplied
- Saquinavir mesylate 200 mg capsules
- Bottles of 270 (NDC 0004-0245-15)
- Saquinavir mesylate 500 mg film-coated tablets
- Bottles of 120 (NDC 0004-0244-51)
Storage
The capsules and tablets should be stored at 25°C (77°F)
Images
Drug Images
{{#ask: Page Name::Saquinavir mesylate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Saquinavir mesylate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Saquinavir mesylate is not a cure for HIV-1 infection and patients may continue to experience illnesses associated with HIV-1 infection, including opportunistic infections. Patients should remain under the care of a physician when using saquinavir mesylate.
Patients should be advised to avoid doing things that can spread HIV-1 infection to others.
- Do not share needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
- Do not breastfeed. We do not know if saquinavir mesylate can be passed to your baby in your breast milk and whether it could harm your baby. Also, mothers with HIV-1 should not breastfeed because HIV-1 can be passed to the baby in the breast milk.
Drug Interactions
saquinavir mesylate may interact with some drugs; therefore, patients should be advised to report to their doctor the use of any other prescription, nonprescription medication, or herbal products, particularly St. John's wort.
PR and QT Interval Prolongation
Patients should be informed that saquinavir mesylate may produce changes in the electrocardiogram (PR interval or QT interval prolongation). Patients should consult their health care provider if they are experiencing symptoms such as dizziness, lightheadedness, or palpitations.
Fat Redistribution
Patients should be informed that redistribution or accumulation of body fat may occur in patients receiving protease inhibitors and that the cause and long-term health effects of these conditions are not known at this time.
Dosing Instructions
Patients should be advised that saquinavir mesylate must be used in combination with ritonavir, which significantly inhibits saquinavir's metabolism to provide increased plasma saquinavir levels.
Patients should be advised that saquinavir mesylate administered with ritonavir should be taken within 2 hours after a full meal [see CLINICAL PHARMACOLOGY (12.3)]. When saquinavir mesylate is taken without food, concentrations of saquinavir in the blood are substantially reduced and may result in no antiviral activity. Patients should be advised of the importance of taking their medication every day, as prescribed, to achieve maximum benefit. Patients should not alter the dose or discontinue therapy without consulting their physician. If a dose is missed, patients should take the next dose as soon as possible. However, the patient should not double the next dose.
Precautions with Alcohol
Alcohol-Saquinavir mesylate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Invirase [1]
Look-Alike Drug Names
There is limited information regarding Saquinavir mesylate Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Saquinavir mesylate |Label Name=Saquinavir mesylate 200 mg.png
}}