Mifepristone
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
TERMINATION OF PREGNANCY
See full prescribing information for complete Boxed Warning.
Mifepristone is a potent antagonist of progesterone and cortisol via the progesterone and glucocorticoid (GR-II) receptors, respectively. The antiprogestational effects will result in the termination of pregnancy. Pregnancy must therefore be excluded before the initiation of treatment with Mifepristone and prevented during treatment and for one month after stopping treatment by the use of a non-hormonal medically acceptable method of contraception unless the patient has had a surgical sterilization, in which case no additional contraception is needed. Pregnancy must also be excluded if treatment is interrupted for more than 14 days in females of reproductive potential.:
|
Overview
Mifepristone is an antagonist of progesterone and cortisol receptors that is FDA approved for the treatment of Cushing's syndrome. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hypertension, peripheral edema, hypokalemia, abdominal pain, decreased apetite, diarrhea, nauseas, vomiting, dizziness, headache, abnormal vaginal bleeding, uterine cramps, hypertrophic endometrial disorder and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hyperglycemia Secondary to Hypercortisolism in Patients with Cushing's Syndrome and Diabetes Mellitus Type-II
- Dosage: 300 mg PO / day taken with meals.
- Maximun dose: 1200 mg/day or 20 mg/kg PO.
- Increases in dose should not occur more frequently than once every 2-4 weeks.
Changes in glucose control, anti-diabetic medication requirements, insulin levels, and psychiatric symptoms may provide an early assessment of response (within 6 weeks) and may help guide early dose titration. Improvements in cushingoid appearance, acne, hirsutism, striae, and body weight occur over a longer period of time and, along with measures of glucose control, may be used to determine dose changes beyond the first 2 months of therapy. Careful and gradual titration of Mifepristone accompanied by monitoring for recognized adverse reactions may reduce the risk of severe adverse reactions. Dose reduction or even dose discontinuation may be needed in some clinical situations. If Mifepristone treatment is interrupted, it should be reinitiated at the lowest dose (300 mg). If treatment was interrupted because of adverse reactions, the titration should aim for a dose lower than the one that resulted in treatment interruption.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mifepristone in adult patients.
Non–Guideline-Supported Use
Dilation of Cervical Canal
- Dosage: 600 mg PO[1].
- Administered 48 hours before procedures which need cervical canal dilation.
Emergency Contraception - Postcoital Contraception
- Dosage:
- 10 mg PO: Emergency contraception during the first 120 hours of a single unprotected sexual intercourse.
- 25 mg PO: Emergency contraception during the first 120 hours of a single unprotected sexual intercourse.
- Both doses prove to have the same efficacy[2].
- 600 mg: Emergency contraception during the first 120 hours of a single unprotected sexual intercourse[3]. In two clinical trials, 600 mg Mifepristone demonstrated to be 100% effective in emergency contraception during the first 120 hours of a single unprotected sexual intercourse[4].
Irregular Periods
- Dosage: 50 mg PO once every 4 weeks[5].
Miscarriage
- Dosage
Refractory Ovarian Cancer
- Dosage: 200 mg/day PO[9].
Uterine leiomyoma
- Dosage: 5 mg PO daily[10].
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Hyperglycemia Secondary to Hypercortisolism in Patients with Cushing's Syndrome and Diabetes Mellitus Type-II
- Dosage: 300 mg PO / day taken with meals.
- Maximun dose: 1200 mg/day or 20 mg/kg PO.
- Increases in dose should not occur more frequently than once every 2-4 weeks.
Changes in glucose control, anti-diabetic medication requirements, insulin levels, and psychiatric symptoms may provide an early assessment of response (within 6 weeks) and may help guide early dose titration. Improvements in cushingoid appearance, acne, hirsutism, striae, and body weight occur over a longer period of time and, along with measures of glucose control, may be used to determine dose changes beyond the first 2 months of therapy. Careful and gradual titration of Mifepristone accompanied by monitoring for recognized adverse reactions may reduce the risk of severe adverse reactions. Dose reduction or even dose discontinuation may be needed in some clinical situations. If Mifepristone treatment is interrupted, it should be reinitiated at the lowest dose (300 mg). If treatment was interrupted because of adverse reactions, the titration should aim for a dose lower than the one that resulted in treatment interruption.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mifepristone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Mifepristone in pediatric patients.
Contraindications
Pregnancy
- Mifepristone is contraindicated in women who are pregnant. Pregnancy must be excluded before the initiation of treatment with Mifepristone or if treatment is interrupted for more than 14 days in females of reproductive potential. Nonhormonal contraceptives should be used during and one month after stopping treatment in all women of reproductive potential.
Drugs Metabolized by CYP3A
Mifepristone is contraindicated in patients taking simvastatin, lovastatin, and CYP3A substrates with narrow therapeutic ranges, such as cyclosporine, dihydroergotamine, ergotamine, fentanyl, pimozide, quinidine, sirolimus, and tacrolimus, due to an increased risk of adverse events.
Corticosteroid Therapy Required for Lifesaving Purposes
Mifepristone is contraindicated in patients who require concomitant treatment with systemic corticosteroids for serious medical conditions or illnesses (e.g., immunosuppression after organ transplantation) because Mifepristone antagonizes the effect of glucocorticoids.
Women with Risk of Vaginal Bleeding or Endometrial Changes
Mifepristone is contraindicated in the following:
- Women with a history of unexplained vaginal bleeding
- Women with endometrial hyperplasia with atypia or endometrial carcinoma
Known Hypersensitivity to Mifepristone
- Mifepristone is contraindicated in patients with prior hypersensitivity reactions to mifepristone or to any of the product components.
Warnings
|
TERMINATION OF PREGNANCY
See full prescribing information for complete Boxed Warning.
Mifepristone is a potent antagonist of progesterone and cortisol via the progesterone and glucocorticoid (GR-II) receptors, respectively. The antiprogestational effects will result in the termination of pregnancy. Pregnancy must therefore be excluded before the initiation of treatment with Mifepristone and prevented during treatment and for one month after stopping treatment by the use of a non-hormonal medically acceptable method of contraception unless the patient has had a surgical sterilization, in which case no additional contraception is needed. Pregnancy must also be excluded if treatment is interrupted for more than 14 days in females of reproductive potential.:
|
Adrenal Insufficiency
- Patients receiving mifepristone may experience adrenal insufficiency. Because serum cortisol levels remain elevated and may even increase during treatment with Mifepristone, serum cortisol levels do not provide an accurate assessment of hypoadrenalism in patients receiving Mifepristone. Patients should be closely monitored for signs and symptoms of adrenal insufficiency, including weakness, nausea, increased fatigue, hypotension, and hypoglycemia. If adrenal insufficiency is suspected, discontinue treatment with Mifepristone immediately and administer glucocorticoids without delay. High doses of supplemental glucocorticoids may be needed to overcome the glucocorticoid receptor blockade produced by mifepristone. Factors considered in deciding on the duration of glucocorticoid treatment should include the long half-life of mifepristone (85 hours).
- Treatment with Mifepristone at a lower dose can be resumed after resolution of adrenal insufficiency. Patients should also be evaluated for precipitating causes of hypoadrenalism (infection, trauma, etc.).
Hypokalemia
- In a study of patients with Cushing's syndrome, hypokalemia was observed in 44% of subjects during treatment with Mifepristone. Hypokalemia should be corrected prior to initiating Mifepristone. During Mifepristone administration, serum potassium should be measured 1 to 2 weeks after starting or increasing the dose of Mifepristone and periodically thereafter. Hypokalemia can occur at any time during Mifepristone treatment. Mifepristone-induced hypokalemia should be treated with intravenous or oral potassium supplementation based on event severity. If hypokalemia persists in spite of potassium supplementation, consider adding mineralocorticoid antagonists.
Vaginal Bleeding and Endometrial Changes
- Being an antagonist of the progesterone receptor, mifepristone promotes unopposed endometrial proliferation that may result in endometrium thickening, cystic dilatation of endometrial glands, and vaginal bleeding. Mifepristone should be used with caution in women who have hemorrhagic disorders or are receiving concurrent anticoagulant therapy. Women who experience vaginal bleeding during Mifepristone treatment should be referred to a gynecologist for further evaluation.
QT Interval Prolongation
- Mifepristone and its metabolites block IKr. Mifepristone prolongs the QTc interval in a dose-related manner. There is little or no experience with high exposure, concomitant dosing with other QT-prolonging drugs, or potassium channel variants resulting in a long QT interval. To minimize risk, the lowest effective dose should always be used.
Exacerbation/Deterioration of Conditions Treated with Corticosteroids
- Use of Mifepristone in patients who receive corticosteroids for other conditions (e.g., autoimmune disorders) may lead to exacerbation or deterioration of such conditions, as Mifepristone antagonizes the desired effects of glucocorticoid in these clinical settings. For medical conditions in which chronic corticosteroid therapy is lifesaving (e.g., immunosuppression in organ transplantation), Mifepristone is contraindicated.
Use of Strong CYP3A Inhibitors
- Mifepristone should be used with extreme caution in patients taking ketoconazole and other strong inhibitors of CYP3A, such as itraconazole, nefazodone, ritonavir, nelfinavir, indinavir, atazanavir, amprenavir, fosamprenavir, boceprevir, clarithromycin, conivaptan, lopinavir, posaconazole, saquinavir, telaprevir, telithromycin, or voriconazole, as these could substantially increase the concentration of mifepristone in the blood. The benefit of concomitant use of these agents should be carefully weighed against the potential risks. Mifepristone should be used in combination with strong CYP3A inhibitors only when necessary, and in such cases the dose should be limited to 300 mg per day.
Pneumocystis jiroveci Infection
- Patients with endogenous Cushing's syndrome are at risk for opportunistic infections such as Pneumocystis jiroveci pneumonia during Mifepristone treatment. Patients may present with respiratory distress shortly after initiation of Mifepristone. Appropriate diagnostic tests should be undertaken and treatment for Pneumocystis jiroveci should be considered.
Potential Effects of Hypercortisolemia
- Mifepristone does not reduce serum cortisol levels. Elevated cortisol levels may activate mineralocorticoid receptors which are also expressed in cardiac tissues. Caution should be used in patients with underlying heart conditions including heart failure and coronary vascular disease.
Adverse Reactions
Clinical Trials Experience
Gastrointestinal Effects
General Effects
Central Nervous System Effects
Musculoeskeletal Effects
Laboratory Findings
- Hypokalemia
- Abnormal thyroid function test (High TSH levels)
- Reduction of HDL-C serum levels
Infectious and Infestations
Metabolism and Nutritional Effects
- Decreased appetite
- Anorexia
Vascular Disorder
Reproductive system and Breast Disorders
Respiratory Effects
Psychiatric Disorders
Postmarketing Experience
There is limited information regarding Mifepristone Postmarketing Experience in the drug label.
Drug Interactions
Based on the long terminal half-life of mifepristone after reaching steady state, at least 2 weeks should elapse after cessation of Mifepristone before initiating or increasing the dose of any interacting concomitant medication.
Drugs Metabolized by CYP3A
- Because Mifepristone is an inhibitor of CYP3A, concurrent use of Mifepristone with a drug whose metabolism is largely or solely mediated by CYP3A is likely to result in increased plasma concentrations of the drug. Discontinuation or dose reduction of such medications may be necessary with Mifepristone co-administration.
- Mifepristone increased the exposure to simvastatin and simvastatin acid significantly in healthy subjects. Concomitant use of simvastatin or lovastatin is contraindicated because of the increased risk of myopathy rhabdomyolysis.
- The exposure of other substrates of CYP3A with narrow therapeutic ranges, such as cyclosporine, dihydroergotamine, ergotamine, fentanyl, pimozide, quinidine, sirolimus, and tacrolimus, may be increased by concomitant administration with Mifepristone. Therefore, the concomitant use of such CYP3A substrates with Mifepristone is contraindicated.
- Other drugs with similar high first pass metabolism in which CYP3A is the primary route of metabolism should be used with extreme caution if co-administered with Mifepristone. The lowest possible dose and/or a decreased frequency of dosing must be used with therapeutic drug monitoring when possible. Use of alternative drugs without these metabolic characteristics is advised when possible with concomitant Mifepristone.
If drugs that undergo low first pass metabolism by CYP3A or drugs in which CYP3A is not the major metabolic route are co-administered with Mifepristone, use the lowest dose of concomitant medication necessary, with appropriate monitoring and follow-up.
CYP3A Inhibitors
- Medications that inhibit CYP3A could increase plasma mifepristone concentrations and dose reduction of Mifepristone may be required.
- Ketoconazole and other strong inhibitors of CYP3A, such as itraconazole, nefazodone, ritonavir, nelfinavir, indinavir, atazanavir, amprenavir and fosamprenavir, boceprevir, clarithromycin, conivaptan, lopinavir, mibefradil, posaconazole, saquinavir, telaprevir, telithromycin, or voriconazole may increase exposure to mifepristone significantly. The clinical impact of this interaction has not been studied. Therefore, extreme caution should be used when these drugs are prescribed in combination with Mifepristone. The benefit of concomitant use of these agents should be carefully weighed against the potential risks. The dose of Mifepristone should be limited to 300 mg and used only when necessary.
- Moderate inhibitors of CYP3A, such as amprenavir, aprepitant, atazanavir, ciprofloxacin, darunavir/ritonavir, diltiazem, erythromycin, fluconazole, fosamprenavir, grapefruit juice, imatinib, or verapamil, should be used with caution when administered in combination with Mifepristone.
CYP3A Inducers
- No medications that induce CYP3A have been studied when co-administered with Mifepristone. Avoid co-administration of Mifepristone and CYP3A inducers such as rifampin, rifabutin, rifapentin, phenobarbital, phenytoin, carbamazepine, and St. John's wort.
Drugs Metabolized by CYP2C8/2C9
- Because Mifepristone is an inhibitor of CYP2C8/CYP2C9, concurrent use of Mifepristone with a drug whose metabolism is largely or solely mediated by CYP2C8/CYP2C9 is likely to result in increased plasma concentrations of the drug. Mifepristone significantly increased exposure of fluvastatin, a typical CYP2C8/CYP2C9 substrate, in healthy subjects. When given concomitantly with Mifepristone, drugs that are substrates of CYP2C8/CYP2C9 (including non-steroidal anti-inflammatory drugs, warfarin, and repaglinide) should be used at the smallest recommended doses, and patients should be closely monitored for adverse effects.
Drugs Metabolized by CYP2B6
- Mifepristone is an inhibitor of CYP2B6 and may cause significant increases in exposure of drugs that are metabolized by CYP2B6 such as bupropion and efavirenz. Since no study has been conducted to evaluate the effect of mifepristone on substrates of CYP2B6, the concomitant use of bupropion and efavirenz should be undertaken with caution.
Use of Hormonal Contraceptives
- Mifepristone is a progesterone-receptor antagonist and will interfere with the effectiveness of hormonal contraceptives. Therefore, non-hormonal contraceptive methods should be used.
Use in Specific Populations
Pregnancy
- Contraindicated in pregnancy. Mifepristone can cause fetal harm when administered to a pregnant woman because the use of Mifepristone results in pregnancy loss. The inhibition of both endogenous and exogenous progesterone by mifepristone at the progesterone receptor results in pregnancy loss. If Mifepristone is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Mifepristone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Mifepristone during labor and delivery.
Nursing Mothers
- Mifepristone is present in human milk of women taking the drug. Because of the potential for serious adverse reactions in nursing infants from Mifepristone, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness of Mifepristone in pediatric patients have not been established.
Geriatic Use
- Clinical studies with Mifepristone did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger people.
Gender
There is no FDA guidance on the use of Mifepristone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Mifepristone with respect to specific racial populations.
Renal Impairment
- The pharmacokinetics of mifepristone in subjects with severe renal impairment (creatinine clearance CrCL < 30 mL/min, but not on dialysis) was evaluated following multiple doses of 1200 mg Mifepristone for 7 days. Mean exposure to mifepristone increased 31%, with similar or smaller increases in metabolite exposure as compared to subjects with normal renal function (CrCL ≥ 90 mL/min). There was large variability in the exposure of mifepristone and its metabolites in subjects with severe renal impairment as compared to subjects with normal renal function (geometric least square mean ratio [CI] for AUC of mifepristone: 1.21 [0.71-2.06]; metabolite 1: 1.43 [0.84-2.44]; metabolite 2: 1.18 [0.64-2.17] and metabolite 3: 1.19 [0.71-1.99]). No change in the initial dose of Mifepristone is needed for renal impairment; the maximum dose should not exceed 600 mg per day.
Hepatic Impairment
- The pharmacokinetics of mifepristone in subjects with moderate hepatic impairment (Child-Pugh Class B) was evaluated in a single- and multiple-dose study (600 mg for 7 days). The pharmacokinetics in subjects with moderate hepatic impairment was similar to those with normal hepatic function. There was large variability in the exposure of mifepristone and its metabolites in subjects with moderate hepatic impairment as compared to subjects with normal hepatic function (geometric least square mean ratio [CI] for AUC of mifepristone: 1.02 [0.59-1.76]; metabolite 1: 0.95 [0.52-1.71]; metabolite 2: 1.37 [0.71-2.62] and metabolite 3: 0.62 [0.33-1.16]). Due to limited information on safety in patients with mild-to-moderate hepatic impairment, the maximum dose should not exceed 600 mg per day. The pharmacokinetics of mifepristone in patients with severe hepatic disease has not been studied. Mifepristone is not recommended in patients with severe hepatic disease.
Females of Reproductive Potential and Males
- Due to its anti-progestational activity, Mifepristone causes pregnancy loss. Exclude pregnancy before the initiation of treatment with Mifepristone or if treatment is interrupted for more than 14 days in females of reproductive potential. Recommend contraception for the duration of treatment and for one month after stopping treatment using a non-hormonal medically acceptable method of contraception. If the patient has had surgical sterilization, no additional contraception is needed.
Immunocompromised Patients
There is no FDA guidance one the use of Mifepristone in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
- Adrenal insufficiency: Patients should be closely monitored for signs and symptoms of adrenal insufficiency.
- Hypokalemia: Hypokalemia should be corrected prior to treatment and monitored for during treatment.
IV Compatibility
There is limited information regarding the compatibility of Mifepristone and IV administrations.
Overdosage
There is no experience with overdosage
Pharmacology
Mechanism of Action
- Mifepristone is a selective antagonist of the progesterone receptor at low doses and blocks the glucocorticoid receptor (GR-II) at higher doses. Mifepristone has high affinity for the GR-II receptor but little affinity for the GR-I (MR, mineralocorticoid) receptor. In addition, mifepristone appears to have little or no affinity for estrogen, muscarinic, histaminic, or monoamine receptors.
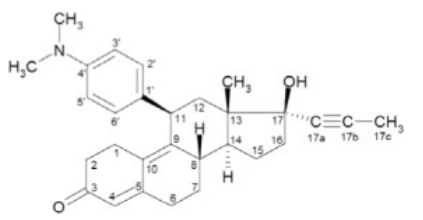
Structure
- The chemical name of mifepristone is 11β-(4-dimethylaminophenyl)-17β-hydroxy-17α-(1-propynyl)-estra-4, 9-dien-3-one. The chemical formula is C29H35NO2; the molecular weight is 429.60, and the structural formula is:
Pharmacodynamics
- Because mifepristone acts at the receptor level to block the effects of cortisol, its antagonistic actions affect the hypothalamic-pituitary-adrenal (HPA) axis in such a way as to further increase circulating cortisol levels while, at the same time, blocking their effects.
- Mifepristone and the three active metabolites have greater affinity for the glucocorticoid receptor (100%, 61%, 48%, and 45%, respectively) than either dexamethasone (23%) or cortisol (9%).
Pharmacokinetics
Absorption
- Following oral administration, time to peak plasma concentrations of mifepristone occurred between 1 and 2 hours following single dose, and between 1 and 4 hours following multiple doses of 600 mg of Mifepristone in healthy volunteers. Mean plasma concentrations of three active metabolites of mifepristone peak between 2 and 8 hours after multiple doses of 600 mg/day, and the combined concentrations of the metabolites exceed that of the parent mifepristone. Exposure to mifepristone is substantially less than dose proportional. Time to steady state is within 2 weeks, and the mean (SD) half-life of the parent mifepristone was 85 (61) hours following multiple doses of 600 mg/day of Mifepristone. Studies evaluating the effects of food on the pharmacokinetics of Mifepristone demonstrate a significant increase in plasma levels of mifepristone when dosed with food. To achieve consistent plasma drug concentrations, patients should be instructed to always take their medication with meals.
Distribution
- Mifepristone is highly bound to alpha-1-acid glycoprotein (AAG) and approaches saturation at doses of 100 mg (2.5 μM) or more. Mifepristone and its metabolites also bind to albumin and are distributed to other tissues, including the central nervous system (CNS). As determined in vitro by equilibrium dialysis, binding of mifepristone and its three active metabolites to human plasma proteins was concentration-dependent. Binding was approximately 99.2% for mifepristone, and ranged from 96.1 to 98.9% for the three active metabolites at clinically relevant concentrations.
Metabolism
- Cytochrome P450 3A4 (CYP3A4) has been shown to be involved in mifepristone metabolism in human liver microsomes. Two of the known active metabolites are the product of demethylation (one monodemethylated and one di-demethylated), while a third active metabolite results from hydroxylation (monohydroxylated).
Elimination and Excretion
- Excretion is primarily (approximately 90%) via the fecal route.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Mifepristone was evaluated for carcinogenicity potential in rats and mice. Rats were dosed for up to two years at doses of 5, 25, and 125 mg/kg of mifepristone. The high dose was the maximum tolerated dose, but exposure at all doses was below exposure at the maximum clinical dose based on AUC comparison. Female rats had a statistically significant increase in follicular cell adenomas/carcinomas and liver adenomas. It is plausible that these tumors are due to drug-induced enzyme metabolism, a mechanism not considered clinically relevant, but studies confirming this mechanism were not conducted with mifepristone. Mice were also tested for up to 2 years at mifepristone doses up to the maximum tolerated dose of 125 mg/kg, which provided exposure below the maximum clinical dose based on AUC. No drug-related tumors were seen in mice. Mifepristone was not genotoxic in a battery of bacterial, yeast, and mammalian in vitro assays, and an in vivo micronucleus study in mice.
- The pharmacological activity of mifepristone disrupts the estrus cycle of adult rats at a dose of 0.3 mg/kg (less than human exposure at the maximum clinical dose, based on body surface area). However, following withdrawal of treatment and subsequent resumption of the estrus cycle, there was no effect on reproductive function when mated. A single subcutaneous dose of mifepristone (up to 100 mg/kg) to rats on the first day after birth did not adversely affect future reproductive function in males or females, although the onset of puberty was slightly premature in dosed females. Repeated doses of mifepristone (1 mg every other day) to neonatal rats resulted in potentially adverse fertility effects, including oviduct and ovary malformations in females, delayed male puberty, deficient male sexual behavior, reduced testicular size, and lowered ejaculation frequency.
Mifepristone was not genotoxic in a battery of bacterial, yeast, and mammalian in vitro assays, and an in vivo micronucleus study in mice.
- The pharmacological activity of mifepristone disrupts the estrus cycle of adult rats at a dose of 0.3 mg/kg (less than human exposure at the maximum clinical dose, based on body surface area). However, following withdrawal of treatment and subsequent resumption of the estrus cycle, there was no effect on reproductive function when mated.
- A single subcutaneous dose of mifepristone (up to 100 mg/kg) to rats on the first day after birth did not adversely affect future reproductive function in males or females, although the onset of puberty was slightly premature in dosed females. Repeated doses of mifepristone (1 mg every other day) to neonatal rats resulted in potentially adverse fertility effects, including oviduct and ovary malformations in females, delayed male puberty, deficient male sexual behavior, reduced testicular size, and lowered ejaculation frequency.
Clinical Studies
Cushing's Syndrome
Results in the Diabetes Cohort
- Patients in the diabetes cohort underwent standard oral glucose tolerance tests at baseline and periodically during the clinical study. Anti-diabetic medications were allowed but had to be kept stable during the trial and patients had to be on stable anti-diabetic regimens prior to enrollment. The primary efficacy analysis for the diabetes cohort was an analysis of responders. A responder was defined as a patient who had a ≥ 25% reduction from baseline in glucose AUC. The primary efficacy analysis was conducted in the modified intent-to-treat population (n=25) defined as all patients who received a minimum of 30 days on Mifepristone. Fifteen of 25 patients (60%) were treatment responders (95% CI: 39%,78%).
- Mean HbA1c was 7.4% in the 24 patients with HbA1c values at baseline and Week 24. For these 24 patients mean reduction in HbA1c was 1.1% (95% CI -1.6, -0.7) from baseline to the end of the trial. Fourteen of 24 patients had above normal HbA1c levels at baseline, ranging between 6.7% and 10.4%; all of these patients had reductions in HbA1c by the end of the study (range -0.4 to -4.4%) and eight of 14 patients (57%) normalized HbA1c levels at trial end. Antidiabetic medications were reduced in 7 of the 15 DM subjects taking antidiabetic medication and remained constant in the others.
Results in the Hypertension Cohort
- There were no changes in mean systolic blood pressure and diastolic blood pressures at the end of the trial relative to baseline in the modified intent-to-treat population (n=21).
Signs and symptoms of Cushing's syndrome in both cohorts
- Individual patients showed varying degrees of improvement in Cushing's syndrome manifestations such as cushingoid appearance, acne, hirsutism, striae, psychiatric symptoms, and excess total body weight. Because of the variability in clinical presentation and variability of response in this open label trial, it is uncertain whether these changes could be ascribed to the effects of Mifepristone.
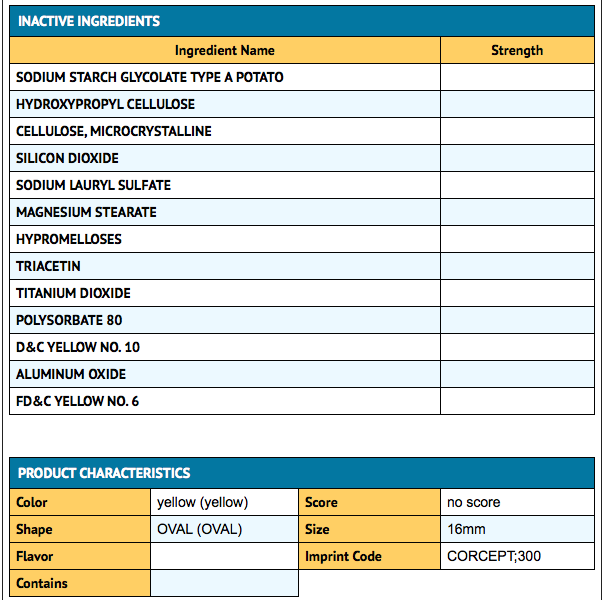
How Supplied
Mifepristone is supplied as a light yellow to yellow, film-coated, oval-shaped tablet debossed with “Corcept” on one side and “300” on the other. Each tablet contains 300 mg of mifepristone. Mifepristone tablets are available in bottles of 28 tablets (NDC 76346-073-01) and bottles of 280 tablets (NDC 76346-073-02).
Storage
Store at controlled room temperature, 25 °C (77 °F); excursions permitted to 15 to 30 ° C (59 – 86 °F).
Images
Drug Images
{{#ask: Page Name::Mifepristone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Mifepristone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
As a part of patient counseling, doctors must review the Mifepristone Medication Guide with every patient.
Importance of Preventing Pregnancy
- Advise patients that Mifepristone will cause termination of pregnancy. Mifepristone is contraindicated in pregnant patients.
- Counsel females of reproductive potential regarding pregnancy prevention and planning with a non-hormonal contraceptive prior to use of Mifepristone and up to one month after the end of treatment.
- Instruct patients to contact their physician immediately if they suspect or confirm they are pregnant.
Precautions with Alcohol
Alcohol-Mifepristone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Mifepristone Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Urquhart DR, Templeton AA (1990). "Mifepristone (RU 486) for cervical priming prior to surgically induced abortion in the late first trimester". Contraception. 42 (2): 191–9. PMID 2085969.
- ↑ Xiao BL, Von Hertzen H, Zhao H, Piaggio G (2002). "A randomized double-blind comparison of two single doses of mifepristone for emergency contraception". Hum Reprod. 17 (12): 3084–9. PMID 12456607.
- ↑ "Comparison of three single doses of mifepristone as emergency contraception: a randomised trial. Task Force on Postovulatory Methods of Fertility Regulation". Lancet. 353 (9154): 697–702. 1999. PMID 10073511.
- ↑ Glasier A (1997). "Emergency postcoital contraception". N Engl J Med. 337 (15): 1058–64. doi:10.1056/NEJM199710093371507. PMID 9321535.
- ↑ Cheng L, Zhu H, Wang A, Ren F, Chen J, Glasier A (2000). "Once a month administration of mifepristone improves bleeding patterns in women using subdermal contraceptive implants releasing levonorgestrel". Hum Reprod. 15 (9): 1969–72. PMID 10966997.
- ↑ Schreiber CA, Creinin MD, Reeves MF, Harwood BJ (2006). "Mifepristone and misoprostol for the treatment of early pregnancy failure: a pilot clinical trial". Contraception. 74 (6): 458–62. doi:10.1016/j.contraception.2006.07.007. PMID 17157102.
- ↑ Cabrol D, Dubois C, Cronje H, Gonnet JM, Guillot M, Maria B; et al. (1990). "Induction of labor with mifepristone (RU 486) in intrauterine fetal death". Am J Obstet Gynecol. 163 (2): 540–2. PMID 2201190.
- ↑ Padayachi T, Moodley J, Norman RJ, Heyns A (1989). "Termination of pregnancy with mifepristone after intra-uterine death. Clinical and hormonal effects". S Afr Med J. 75 (11): 540–2. PMID 2658142.
- ↑ Rocereto TF, Saul HM, Aikins JA, Paulson J (2000). "Phase II study of mifepristone (RU486) in refractory ovarian cancer". Gynecol Oncol. 77 (3): 429–32. doi:10.1006/gyno.2000.5789. PMID 10831354.
- ↑ Eisinger SH, Meldrum S, Fiscella K, le Roux HD, Guzick DS (2003). "Low-dose mifepristone for uterine leiomyomata". Obstet Gynecol. 101 (2): 243–50. PMID 12576246.
{{#subobject:
|Label Page=Mifepristone |Label Name=28.png
}}
{{#subobject:
|Label Page=Mifepristone |Label Name=280.png
}}