Phosphorous acid
|
WikiDoc Resources for Phosphorous acid |
|
Articles |
|---|
|
Most recent articles on Phosphorous acid Most cited articles on Phosphorous acid |
|
Media |
|
Powerpoint slides on Phosphorous acid |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Phosphorous acid at Clinical Trials.gov Trial results on Phosphorous acid Clinical Trials on Phosphorous acid at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Phosphorous acid NICE Guidance on Phosphorous acid
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Phosphorous acid Discussion groups on Phosphorous acid Patient Handouts on Phosphorous acid Directions to Hospitals Treating Phosphorous acid Risk calculators and risk factors for Phosphorous acid
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Phosphorous acid |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: Henry A. Hoff
Overview
Phosphorous acid is the compound described by the formula H3PO3. It is one of the oxoacids of phosphorus, other important members being phosphoric acid (H3PO4) and hypophosphorous acid (H3PO2). Note that only the reduced phosphorus compounds are spelled with an "ous" ending. Other names for this acid are orthophosphorous acid and dihydroxyphosphine oxide.
HP(O)(OH)2 is the product of the hydrolysis of its acid anhydride, P4O6:
- P4O6 + 6 H2O → 4 HP(O)(OH)2
An analogous relationship connects H3PO4 and P4O10.
Tautomerization
H3PO3 is better described with the structural formula HP(O)(OH)2. This species exists in equilibrium with a minor tautomer P(OH)3. The latter is called phosphorous acid.[1]
P(OH)3 (IUPAC: phosphorous acid) has CAS number 10294-56-1 and CID: 107909[1]. It is sometimes called phosphorus acid or orthophosphorous acid. It has been shown to be a stable tautomer.[2][3]
The dihydroxy form, HP(O)(OH)2, is called phosphonic acid. Many of the reduced phosphorus acids are subject to similarly complicated equilibria involving shifts of H between O and P. In the solid state, HP(O)(OH)2 is tetrahedral with one shorter P=O bond of 148 pm and two longer P-O(H) bonds of 154 pm.
Preparation
Although commercially available, the acid is most commonly prepared by hydrolysis of phosphorus trichloride with water or steam:
- PCl3 + 3 H2O → HP(O)(OH)2 + 3 HCl
Potassium phosphite is a convenient precursor to phosphorous acid:
- K2HPO3 + 2 HCl → 2 KCl + H3PO3
In practice aqueous potassium phosphite is treated with excess hydrochloric acid. By concentrating the solution and precipitations with alcohols, the pure acid can be separated from the salt.
Polymerization
An oligophosphorous acid of the phosphonic acid tautomer refers to a few molecules of phosphorous acid condensed into a molecule with the loss of water.
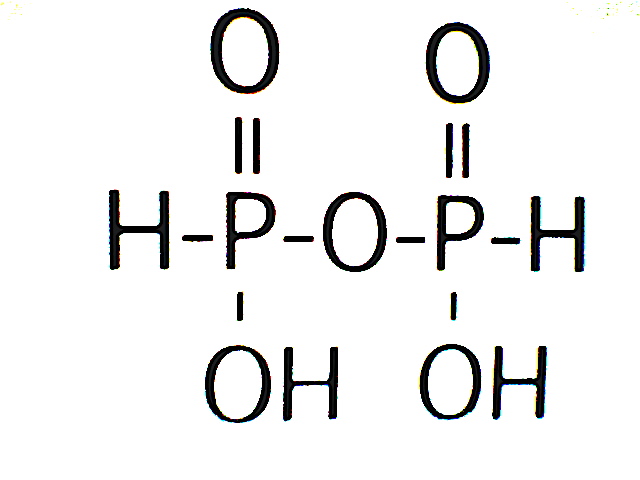
A general formula for such oligophosphorous acids is (HPO)nOn-1(OH)2, where n = 2, 3, 4, etc., oligo-. A polyphosphorous acid can have dozens of such phosphorous acid units condensed in a row with the loss of H2O for each unit added on.
For the phosphorous acid tautomer, an oligophosphorous acid also refers to a few molecules condensed into a molecule with the loss of H2O as each unit of P(OH)3 is added on, but the general formula differs:
(HO)2PO[P(OH)O]n-2P(OH)2,
where n = 2, 3, 4, etc., oligo-. Here for both tautomers the repeat unit is (HPO2)n-2.

Again, a polyphosphorous acid can have dozens of units condensed in a row. Regardless of the value of n, both polyphosphonic acid and polyphosphorous acid have the same chemical formula for any specific n, e.g., triphosphosphonic acid is H5P3O7 and triphosphorous acid is H5P3O7 for n=3.
In oligophosphorous acids of sufficient size, there are multiple OH that can result in the condensation of a cyclophosphorous acid that does not have multiple (HPO3) metaphosphoric acid units.
However, the usual referral to a cyclophosphorous acid (cyclophosphonates or cyclophosphites) may be misnomers wherein the cyclic portion is carbon-based with a phosphorous acid side chain of one or more molecules, or one or a limited number of either of the two tautomers included in the ring but as a minority contributor. For example the effect of varying ring size on the phosphonate-phosphite tautomerism of cyclophosphorous acids has been shown.[4] But the cyclophosphorous acids are biheteroorganic.
Branching can also occur in either oligophosphorous or polyphosphorous tautomer. These are ultraoligophosphorous or ultrapolyphosphorous acids, ultraoligophosphonates and ultrapolyphosphonates, or ultraoligophosphites and ultrapolyphosphites, respectively for the phosphonic and phosphorous tautomers.
Acid-base properties
Phosphorous acid is a diprotic acid, since the hydrogen bonded directly to the central phosphorus atom is not readily ionizable. Chemistry examinations often test students' appreciation of the fact that all three hydrogen atoms are not acidic under aqueous conditions, in contrast with phosphoric acid. HP(O)2(OH)− is a moderately strong acid.
- HP(O)(OH)2 → HP(O)2(OH)− + H+ pKa = 1.3[5]
- HP(O)2(OH)− → HPO32− + H+ pKa = 6.7
The monodeprotonated species, HP(O)2(OH)− is called the phosphite ion.
The IUPAC (mostly organic) name is phosphonic acid. This nomenclature is commonly reserved for substituted derivatives, that is, organic group bonded to phosphorus, not simply an ester. For example, (CH3)PO(OH)2 is "methylphosphonic acid", which may of course form "methylphosphonate" esters.
Both phosphorous acid and its deprotonated forms are good reducing agents, although not necessarily quick to react. They are oxidized to phosphoric acid or its salts. It reduces solutions of noble metal cations to the metals.
Uses
Conversion to phosphine
Phosphine, being a flammable and toxic gas, is inconvenient to store. Fortunately this useful species is readily prepared by thermal decomposition of phosphorous acid, which degrades at about 180°C:
- 4 HP(O)(OH)2 → PH3 + 3 H3PO4
Since phosphoric acid is a syrupy non-volatile liquid, the gaseous PH3 is readily separated.
In agriculture
A large quantity of phosphorous acid is used as phosphatic fertilizer. [6] Pure phosphorous acid is also used for preparing phosphite salts, such as monopotassium phosphite or aluminum phosphonite. These salts, as well as aqueous solutions of pure phosphorous acid, have shown effectiveness in controlling a variety of microbial plant diseases—in particular, treatment using either trunk injection or foliar sprays containing phosphorous acid salts is indicated in response to infections by phytophthora and pythium-type plant pathogens (both within class oomycetes, known as water molds), such as dieback/root rot and downy mildew.[7] Anti-microbial products containing salts of phosphorous acid are marketed in Australia as 'Yates Anti-Rot'; and in the United States of America, for example, aluminum salts of phosphorous acid (known generically as 'Fosetyl-Al') are sold under the trade name 'Aliette'.[8][9]
As a chemical reagent
Phosphorous acid is used in chemical reactions as a reducing agent that is somewhat less vigorous than the related hypophosphorous acid.[10]
References
- ↑ 1.0 1.1 "Phosphorous acid - PubChem Public Chemical Database".
- ↑ Xi C, Liu Y, Lai C, Zhou L (2004). "Synthesis of molybdenum complex with novel P(OH)3 ligand based on the one-pot reaction of Mo(CO)6 with HP(O)(OEt)2 and water". Inorgn Chem Commun. 7 (11): 1202–4. doi:10.1016/j.inoche.2004.09.012.
- ↑ Sokolov MN, Chubarova EV, Kovalenko KA, Mironov IV, Virovets1 AV, Peresypkina EV, Fedin VP (2005). "Stabilization of tautomeric forms P(OH)3 and HP(OH)2 and their derivatives by coordination to palladium and nickel atoms in heterometallic clusters with the Mo3MQ44+ core (M = Ni, Pd; Q = S, Se)". Russ Chem Bull. 54, (3): 615–22. doi:10.1007/s11172-005-0296-1.
- ↑ Gladyshev EN, Bayushkin PY, Sokolov VS (1978). "Some one-electron oxidation reactions of biheteroorganic derivatives with Ge-Hg and Ge-Li groupings". Russ Chem Bull. 27 (3): 592–5. doi:10.1007/BF00923949. Unknown parameter
|month=ignored (help) - ↑ CRC Handbook of Chemistry and Physics, 87th Ed. 8-42
- ↑ Allison E. McDonald; Bruce R. Grant; William C. Plaxton (2001). "Phosphite (Phosphorous acid ): Its Relevance in the Environment and Agriculture and Influence on Plant Phosphate Starvation Respose". Journal of Plant Nutrition. 24 (10): 1505–1519. doi:10.1081/PLN-100106017.
- ↑ Organic Labs. Product label for 'Exel LG,' Retrieved April 9, 2007.
- ↑ Yates, a Division of Orica Australia Pty Ltd. “MSDS ('Yates Anti Rot Phosacid Systemic Fungicide').” Version 1. SH&E Shared Services, Orica. Homebush, NSW (Australia): April 4, 2005 (retrieved from www.orica.com April 9, 2007).
- ↑ US EPA. “Fosetyl-Al (Aliette): Reregistration Eligibility Decision (RED) Fact Sheet.” Office of Pesticide Programs, US EPA. Washington, DC (USA): 1994 (retrieved from www.epa.gov April 9, 2007).
- ↑ “Phosphorous acid.” The American Heritage® Dictionary of the English Language, 4th ed. Boston: Houghton Mifflin, 2000 (retrieved from www.bartleby.com April 9, 2007).
Additional Resources
- Holleman, A. F.; Wiberg, E. “Inorganic Chemistry.” Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- D. E. C. Corbridge. “Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology.” 5th ed. Elsevier: Amsterdam. ISBN 0-444-89307-5.
cs:Kyselina fosforitá de:Phosphonsäure eo:Fosfonata acido it:Acido fosforoso