Ceftizoxime
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ceftizoxime is a 3rd Generation Cephalosporin that is FDA approved for the treatment of Lower Respiratory Tract Infections, Urinary Tract Infections, Gonorrhea, Pelvic Inflammatory Disease, IntraAbdominal Infections, Septicemia, Skin and Skin Structure Infections, Bone and Joint Infections, Meningitis,. Common adverse reactions include Injection site pain, Pruritus, Rash, Increased liver enzymes, Fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Cefizox (ceftizoxime for injection, USP) is indicated in the treatment of infections due to susceptible strains of the microorganisms listed below.
- Lower Respiratory Tract Infections caused by Klebsiella spp.; Proteus mirabilis; Escherichia coli; Haemophilus influenzae including ampicillinresistant strains; Staphylococcus aureus (penicillinase and nonpenicillinaseproducing); Serratia spp.; Enterobacter spp.; Bacteroides spp.; and Streptococcus spp. including S. pneumoniae, but excluding enterococci.
- Urinary Tract Infections caused by Staphylococcus aureus (penicillinase and nonpenicillinaseproducing); Escherichia coli; Pseudomonas spp. including P.aeruginosa; Proteus mirabilis; P. vulgaris; Providencia rettgeri (formerly Proteus rettgeri) and Morganella morganii (formerly Proteus morganii); Klebsiella spp.; Serratia spp. including S. marcescens; and Enterobacter spp.
- Gonorrhea including uncomplicated cervical and urethral gonorrhea caused by Neisseria gonorrhoeae.
- Pelvic Inflammatory Disease caused by Neisseria gonorrhoeae, Escherichia coli or Streptococcus agalactiae. NOTE: Ceftizoxime, like other cephalosporins, has no activity against Chlamydia trachomatis. Therefore, when cephalosporins are used in the treatment of patients with pelvic inflammatory disease and C. trachomatis is one of the suspected pathogens, appropriate antichlamydial coverage should be added.
- IntraAbdominal Infections caused by Escherichia coli; Staphylococcusepidermidis; Streptococcus spp. (excluding enterococci); Enterobacter spp.; Klebsiella spp.; Bacteroides spp. including B. fragilis; and anaerobic cocci, including Peptococcus spp. and Peptostreptococcus spp.
- Septicemia caused by Streptococcus spp. including S. pneumoniae (but excluding enterococci); Staphylococcus aureus (penicillinase and nonpenicillinaseproducing); Escherichia coli; Bacteroides spp. including B. fragilis; Klebsiella spp.; and Serratia spp.
- Skin and Skin Structure Infections caused by Staphylococcus aureus (penicillinase and nonpenicillinaseproducing); Staphylococcus epidermidis; Escherichia coli; Klebsiella spp.; Streptococcus spp. including Streptococcus pyogenes (but excluding enterococci); Proteus mirabilis; Serratia spp.; Enterobacter spp.; Bacteroides spp. including B. fragilis; and anaerobic cocci, including Peptococcus spp. and Peptostreptococcus spp.
- Bone and Joint Infections caused by Staphylococcus aureus (penicillinase and nonpenicillinaseproducing); Streptococcus spp. (excluding enterococci); Proteusmirabilis; Bacteroides spp.; and anaerobic cocci, including Peptococcus spp. and Peptostreptococcus spp.
- Meningitis caused by Haemophilus influenzae. Cefizox has also been used successfully in the treatment of a limited number of pediatric and adult cases of meningitis caused by Streptococcus pneumoniae.
- Cefizox has been effective in the treatment of seriously ill, compromised patients, including those who were debilitated, immunosuppressed, or neutropenic.
- Infections caused by aerobic gramnegative and by mixtures of organisms resistant to other cephalosporins, aminoglycosides, or penicillins have responded to treatment with Cefizox.
- Because of the serious nature of some urinary tract infections due to P. aeruginosa and because many strains of Pseudomonas species are only moderately susceptible to Cefizox, higher dosage is recommended. Other therapy should be instituted if the response is not prompt.
- Susceptibility studies on specimens obtained prior to therapy should be used to determine the response of causative organisms to Cefizox. Therapy with Cefizox may be initiated pending results of the studies; however, treatment should be adjusted according to study findings. In serious infections, Cefizox has been used
- concomitantly with aminoglycosides. Before using Cefizox
- concomitantly with other antibiotics, the prescribing information for those agents
- should be reviewed for contraindications, warnings, precautions, and adverse reactions. Renal function should be carefully monitored.
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of Cefizox and other antibacterial drugs, Cefizox should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Dosage
- The usual adult dosage is 1 or 2 grams of Cefizox (ceftizoxime for injection, USP) every 8 to 12 hours. Proper dosage and route of administration should be determined by the condition of the patient, severity of the infection, and susceptibility of the causative organisms.
- Because of the serious nature of urinary tract infections due to P. aeruginosa and because many strains of Pseudomonas species are only moderately susceptible to Cefizox, higher dosage is recommended. Other therapy should be instituted if the response is not prompt.
- A single, 1 gram IM dose is the usual dose for treatment of uncomplicated gonorrhea.
- The IV route may be preferable for patients with bacterial septicemia, localized parenchymal abscesses (such as intraabdominal abscess), peritonitis, or other severe or lifethreatening infections.
- In those with normal renal function, the IV dosage for such infections is 2 to 12 grams of Cefizox (ceftizoxime for injection, USP) daily. In conditions such as bacterial septicemia, 6 to 12 grams/day may be given initially by the IV route for several days, and the dosage may then be gradually reduced according to clinical response and laboratory findings.
- Dosage may be increased to a total daily dose of 200 mg/kg (not to exceed the maximum adult dose for serious infection).
Impaired Renal Function
- Modification of Cefizox dosage is necessary in patients with impaired renal function. Following an initial loading dose of 500 mg-1 gram IM or IV, the maintenance dosing schedule shown below should be followed. Further dosing should be determined by therapeutic monitoring, severity of the infection, and susceptibility of the causative organisms.
- When only the serum creatinine level is available, creatinine clearance may be calculated from the following formula. The serum creatinine level should represent current renal function at the steady state.
Males
- Clcr =Weight (kg) x (140 age)
- 72 x serum creatinine
- (mg/100 mL)
- Females are 0.85 of the calculated clearance values for males.
- In patients undergoing hemodialysis, no additional supplemental dosing is required following hemodialysis; however, dosing should be timed so that the patient receives the dose (according to the table below) at the end of the dialysis.
Preparation of Parenteral Solution
- RECONSTITUTION
- IM Administration: Reconstitute with Sterile Water for Injection. SHAKE WELL.
- These solutions of Cefizox are stable 24 hours at room temperature or 96 hours if refrigerated (5ºC).
- Parenteral drug products should be inspected visually for particulate matter prior to administration. If particulate matter is evident in reconstituted fluids, then the drug solution should be discarded. Reconstituted solutions may range from yellow to amber without changes in potency.
- Piggyback Vials: Reconstitute with 50 to 100 mL of Sodium Chloride Injection or any other IV solution listed below.
- SHAKE WELL.
- Administer with primary IV fluids, as a single dose. These Piggyback vial solutions of Cefizox are stable 24 hours at room temperature or 96 hours if refrigerated (5ºC).
- A solution of 1 gram Cefizox in 13 mL Sterile Water for Injection is isotonic.
- IM Injection
- Inject well within the body of a relatively large muscle. Aspiration is necessary to avoid inadvertent injection into a blood vessel. When administering 2 gram IM doses, the dose should be divided and given in different large muscle masses.
- IV Administration
- Direct (bolus) injection, slowly over 3 to 5 minutes, directly or through tubing for patients receiving parenteral fluids (see list below). Intermittent or continuous infusion, dilute reconstituted Cefizox in 50 to 100 mL of one of the following solutions:
- Sodium Chloride Injection
- 5% or 10% Dextrose Injection
- 5% Dextrose and 0.9%, 0.45%, or 0.2% Sodium Chloride Injection
- Ringer’s Injection
- Lactated Ringer’s Injection
- Invert Sugar 10% in Sterile Water for Injection
- 5% Sodium Bicarbonate in Sterile Water for Injection
- 5% Dextrose in Lactated Ringer’s Injection (only when reconstituted with 4% Sodium Bicarbonate Injection)
- In these fluids, Cefizox is stable 24 hours at room temperature or 96 hours if refrigerated (5ºC).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ceftizoxime in adult patients.
Non–Guideline-Supported Use
- Bacterial infectious disease - Cancer[1][2]
- Gonorrhea, Disseminated
- Postoperative infection; Prophylaxis[3]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Dosage
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ceftizoxime in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ceftizoxime in pediatric patients.
Contraindications
- Cefizox (ceftizoxime for injection, USP) is contraindicated in patients who have known allergy to the drug.
Warnings
- BEFORE THERAPY WITH CEFIZOX IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFIZOX, OTHER CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLINSENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS HYPERSENSITIVITY AMONG BETALACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEFIZOX OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, IV FLUIDS, IV ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
- Pseudomembranous colitis has been reported with nearly all antibacterial agents, including ceftizoxime, and may range in severity from mild to life threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
- Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is a primary cause of “antibioticassociated” colitis.
- After the diagnosis of pseudomembranous colitis has been established, appropriate therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against Clostridium difficile colitis.
Precautions
General
- As with all broadspectrum antibiotics, Cefizox (ceftizoxime for injection, USP) should be prescribed with caution in individuals with a history of gastrointestinal disease, particularly colitis.
- Although Cefizox has not been shown to produce an alteration in renal function, renal status should be evaluated, especially in seriously ill patients receiving maximum dose therapy. As with any antibiotic, prolonged use may result in overgrowth of nonsusceptible organisms. Careful observation is essential; appropriate measures should be taken if superinfection occurs.
- Cephalosporins may be associated with a fall in prothrombin activity. Those at risk include patients with renal or hepatic impairment, or poor nutritional state, as well as patients receiving a protracted course of antimicrobial therapy, and patients previously stabilized on anticoagulant therapy. Prothrombin time should be monitored in patients at risk and exogenous vitamin K administered as indicated.
- Prescribing Cefizox in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Adverse Reactions
Clinical Trials Experience
- Cefizox® (ceftizoxime for injection, USP) is generally well tolerated. The most frequent adverse reactions (greater than 1% but less than 5%) are:
Hypersensitivity
Hepatic
- Transient elevation in AST (SGOT), ALT (SGPT), and alkaline phosphatase.
Hematologic
- Transient eosinophilia, thrombocytosis. Some individuals have developed a positive Coombs test.
Local
- Injection site--Burning, cellulitis, phlebitis with IV administration, pain, induration, tenderness, paresthesia.
- The less frequent adverse reactions (less than 1%) are:
Hypersensitivity
- Numbness and anaphylaxis have been reported rarely.
Hepatic
- Elevation of bilirubin has been reported rarely.
Renal
- Transient elevations of BUN and creatinine have been occasionally observed with Cefizox.
Hematologic
- Anemia, including hemolytic anemia with occasional fatal outcome, leukopenia, neutropenia, and thrombocytopenia have been reported rarely.
Urogenital
- Vaginitis has occurred rarely.
Gastrointestinal
- Symptoms of pseudomembranous colitis can appear during or after antibiotic treatment.
- In addition to the adverse reactions listed above which have been observed in patients treated with ceftizoxime, the following adverse reactions and altered laboratory tests have been reported for cephalosporinclass antibiotics:
- StevensJohnson syndrome, erythema multiforme, toxic epidermal necrolysis, serumsickness like reaction, toxic nephropathy, aplastic anemia, hemorrhage, prolonged prothrombin time, elevated LDH, pancytopenia, and agranulocytosis.
- Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment, when the dosage was not reduced. If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Ceftizoxime in the drug label.
Drug Interactions
- Although the occurrence has not been reported with Cefizox, nephrotoxicity has been reported following concomitant administration of other cephalosporins and aminoglycosides.
Use in Specific Populations
Pregnancy
- Reproduction studies performed in rats and rabbits have revealed no evidence of impaired fertility or harm to the fetus due to Cefizox. There are, however, no adequate and wellcontrolled studies in pregnant women. Because animal reproduction studies are not always predictive of human effects, this drug should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ceftizoxime in women who are pregnant.
Labor and Delivery
- Safety of Cefizox use during labor and delivery has not been established.
Nursing Mothers
- Cefizox is excreted in human milk in low concentrations. Caution should be exercised when Cefizox is administered to a nursing woman.
Pediatric Use
- Safety and efficacy in pediatric patients from birth to six months of age have not been established. In pediatric patients six months of age and older, treatment with Cefizox has been associated with transient elevated levels of eosinophils, AST (SGOT), ALT (SGPT), and CPK (creatine phosphokinase). The CPK elevation may be related to IM administration.
The potential for the toxic effect in pediatric patients from chemicals that may leach from the singledose IV preparation in plastic has not been determined.
Geriatic Use
- Clinical studies of ceftizoxime did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Ceftizoxime with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ceftizoxime with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ceftizoxime in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Ceftizoxime in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ceftizoxime in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ceftizoxime in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
- Prothrombin time should be monitored in patients at risk and exogenous vitamin K administered as indicated.
IV Compatibility
There is limited information regarding IV Compatibility of Ceftizoxime in the drug label.
Overdosage
There is limited information regarding Overdose of Ceftizoxime in the drug label.
Pharmacology
| |
Ceftizoxime
| |
| Systematic (IUPAC) name | |
| (6R,7R)-7-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | |
| Identifiers | |
| CAS number | 68401-82-1 |
| ATC code | J01 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 383.405 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- The bactericidal action of ceftizoxime results from inhibition of cellwall synthesis. Ceftizoxime is highly resistant to a broad spectrum of betalactamases (penicillinase and cephalosporinase), including Richmond types I, II, III, TEM, and IV, produced by both aerobic and anaerobic grampositive and gramnegative organisms.
Structure
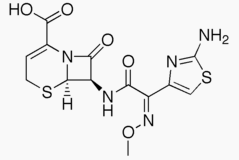
- Cefizox® (ceftizoxime for injection, USP) is a sterile, semisynthetic, broadspectrum, betalactamase resistant cephalosporin antibiotic for parenteral (IV, IM) administration. It is the sodium salt of [6R6a,7β(Z)]7(2,3dihydro2imino4 thiazolyl) (methoxyimino) acetyl amino8oxo5thia1azabicyclo [4.2.0] oct2ene2carboxylic acid. Its sodium content is approximately 60 mg (2.6 mEq) per gram of ceftizoxime activity.
- It has the following structural formula:
- Ceftizoxime for injection, USP is a white to pale yellow crystalline powder.
- Cefizox is supplied in vials equivalent to 500 mg, 1 gram or 2 grams of ceftizoxime, and in Piggyback Vials for IV admixture equivalent to 1 gram or 2 grams of ceftizoxime.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Ceftizoxime in the drug label.
Pharmacokinetics
- The table below demonstrates the serum levels and duration of Cefizox (ceftizoxime for injection, USP) following IM administration of 500 mg and 1 gram doses, respectively, to normal volunteers.
- A serum halflife of approximately 1.7 hours was observed after IV or IM administration.
- Cefizox is 30% protein bound.
- Cefizox is not metabolized, and is excreted virtually unchanged by the kidneys in 24 hours. This provides a high urinary concentration. Concentrations greater than 6000 μg/mL have been achieved in the urine by 2 hours after a 1 gram dose of Cefizox intravenously. Probenecid slows tubular secretion and produces even higher serum levels, increasing the duration of measurable serum concentrations.
- Cefizox achieves therapeutic levels in various body fluids, e.g., cerebrospinal fluid (in patients with inflamed meninges), bile, surgical wound fluid, pleural fluid, aqueous humor, ascitic fluid, peritoneal fluid, prostatic fluid and saliva, and in the following body tissues: heart, gallbladder, bone, biliary, peritoneal, prostatic, and uterine.
- In clinical experience to date, no disulfiramlike reactions have been reported with Cefizox.
Microbiology
- The bactericidal action of ceftizoxime results from inhibition of cellwall synthesis. Ceftizoxime is highly resistant to a broad spectrum of betalactamases (penicillinase and cephalosporinase), including Richmond types I, II, III, TEM, and IV, produced by both aerobic and anaerobic grampositive and gramnegative organisms. Ceftizoxime has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections described in the INDICATIONS AND USAGE section:
Aerobic Gram-Positive Microorganisms
- Staphylococcus aureus (including penicillinase producing strains)
- NOTE: Methicillinresistant staphylococci are resistant to cephalosporins, including ceftizoxime.
- Staphylococcus epidermidis (including penicillinase producing strains)
- NOTE: A streptococcal isolate that is susceptible to penicillin can be considered susceptible to ceftizoxime.
- NOTE: Ceftizoxime is usually inactive against most strains of Enterococcus faecalis.
Aerobic Gram-Negative Microorganisms
- Haemophilus influenzae (including ampicillinresistant strains)
Anaerobic Microorganisms
- The following in vitro data are available, but their clinical significance is unknown. At least 90% of the following microorganisms exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for ceftizoxime. However, the safety and effectiveness of ceftizoxime in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.
Aerobic Gram-Negative Microorganisms
Susceptibility Testing Methods:
Dilution techniques:
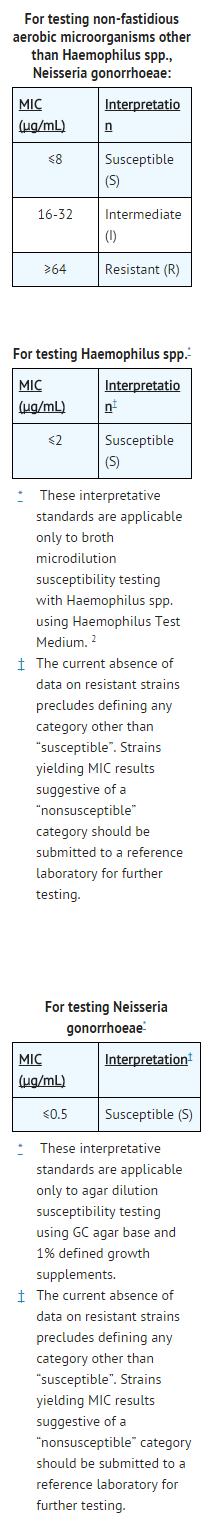
- Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method1 (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of ceftizoxime powder. The MIC values should be interpreted according to the following criteria:
- A report of “Susceptible” indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of “Intermediate” indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone, which prevents small-uncontrolled technical factors from causing major discrepancies in interpretation. A report of “Resistant” indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable, other therapy should be selected.
- Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard ceftizoxime powder should provide the following MIC values:
Diffusion Techniques:
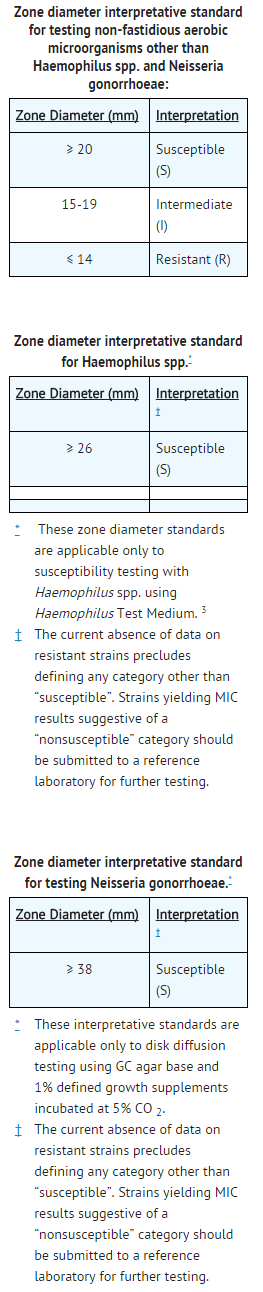
- Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 30-μg ceftizoxime to test the susceptibility of microorganisms to ceftizoxime.
- Reports from the laboratory providing results of the standard single-disk susceptibility test with a 30-μg ceftizoxime disk should be interpreted according to the following criteria:
- Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for ceftizoxime.
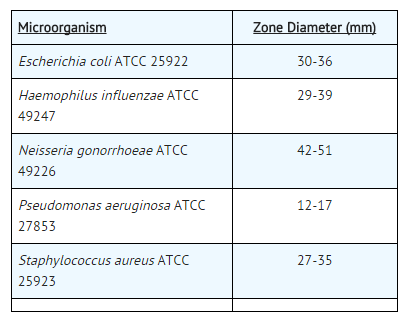
- As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 30-μg ceftizoxime disk should provide the following zone diameters in these laboratory test quality control strains:
Anaerobic Techniques:
- For anaerobic bacteria, the susceptibility to ceftizoxime as MICs can be determined by standardized test methods. Agar dilution results can vary widely when using ceftizoxime. It is recommended that broth microdilution method be used when possible.3 The MIC values obtained should be interpreted according to following criteria:
- Interpretation is identical to that described in Susceptibility Testing: Dilution Techniques.
- As with other susceptibility techniques, the use of laboratory control microorganisms is required to control the technical aspects of the laboratory standardized procedures. Standardized ceftizoxime powder should provide the following MIC values:
Susceptibility Testing for Pseudomonas in Urinary Tract Infections
- Most strains of Pseudomonas aeruginosa are moderately susceptible to ceftizoxime.
- Ceftizoxime achieves high levels in the urine (greater than 6000 μg/mL at 2 hours with
1 gram IV) and, therefore, the following zone sizes should be used when testing
ceftizoxime for treatment of urinary tract infections caused by Pseudomonas
aeruginosa.
- Susceptible organisms produce zones of 20 mm or greater, indicating that the
test organism is likely to respond to therapy.
- Organisms that produce zones of 11 to 19 mm are expected to be susceptible
when the infection is confined to the urinary tract (in which high antibiotic levels
are attained).
- Resistant organisms produce zones of 10 mm or less, indicating that other
therapy should be selected.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Longterm studies in animals to evaluate the carcinogenic potential of ceftizoxime have not been conducted.
- In an in vitro bacterial cell assay (i.e., Ames test), there was no evidence of mutagenicity at ceftizoxime concentrations of 0.0010.5 mcg/plate. Ceftizoxime did not produce increases in micronuclei in the in vivo mouse micronucleus test when given to animals at doses up to 7500 mg/kg, approximately six times greater than the maximum human daily dose on a mg/M2 basis.
- Ceftizoxime had no effect on fertility when administered subcutaneously to rats at daily doses of up to 1000 mg/kg/day, approximately two times the maximum human daily dose on a mg/M2 basis. Ceftizoxime produced no histological changes in the sexual organs of male and female dogs when given intravenously for thirteen weeks at a dose of 1000 mg/kg/day, approximately five times greater than the maximum human daily dose on a mg/M2 basis.
Clinical Studies
There is limited information regarding Clinical Studies of Ceftizoxime in the drug label.
How Supplied
- Cefizox® (ceftizoxime for injection, USP)
- NDC 0469725001 Product No. 725001
- Equivalent to 500 mg ceftizoxime in 10 mL, singledose, fliptop vials, individually
packaged
- NDC 0469725101 Product No. 725101
- Equivalent to 1 gram ceftizoxime in 20 mL, singledose, fliptop vials, individually
packaged
- NDC 0469725201 Product No. 725201
- Equivalent to 1 gram ceftizoxime in 100 mL, singledose, Piggyback, fliptop vials, packaged in tens
- NDC 0469725302 Product No. 725302
- Equivalent to 2 grams ceftizoxime in 20 mL, singledose, fliptop vials, individually packaged
- NDC 0469725402 Product No. 725402
- Equivalent to 2 grams ceftizoxime in 100 mL, singledose, Piggyback, fliptop vials, packaged in tens
Storage
- Unreconstituted Cefizox should be protected from excessive light, and stored at controlled room temperature (59º86ºF) in the original package until used.
Images
Drug Images
{{#ask: Page Name::Ceftizoxime |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
Ingredients and Appearance
{{#ask: Label Page::Ceftizoxime |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be counseled that antibacterial drugs including Cefizox should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Cefizox is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Cefizox or other antibacterial drugs in the future.
Precautions with Alcohol
- Alcohol-Ceftizoxime interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Cefizox®[4]
Look-Alike Drug Names
There is limited information regarding Ceftizoxime Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Rolston KV, Jones PG, Fainstein V, Elting L, Bodey GP (1987). "Ceftizoxime plus ticarcillin: double beta-lactam therapy for infections in cancer patients". J Antimicrob Chemother. 19 (3): 367–71. PMID 3471752.
- ↑ Lawson RD, Baskin RC (1982). "Ceftizoxime treatment of infection in neutropenic patients with malignancies". J Antimicrob Chemother. 10 Suppl C: 159–65. PMID 6296001.
- ↑ Ridley PD, Sagar S (1988). "Cephalosporin prophylaxis in abdominal surgery". Br J Clin Pract. 42 (8): 337–42. PMID 3061436.
- ↑ "Ceftizoxime sodium".