Cardiac tamponade surgery
|
Cardiac tamponade Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Cardiac tamponade surgery On the Web |
|
American Roentgen Ray Society Images of Cardiac tamponade surgery |
|
Risk calculators and risk factors for Cardiac tamponade surgery |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Priyantha Ranaweera [2], Mohammed A. Sbeih, M.D. [3] Ramyar Ghandriz MD[4]
Overview
Percutaneous pericardiocentesis is a procedure where fluid is aspirated from the pericardium (the sac enveloping the heart) using a needle via a percutaneous approach. Pericardiocentesis can provide a diagnostic sampling of pericardial fluid and can be used as a therapeutic maneuver to evacuate pericardial fluid and lower the pericardial pressure.
Indications
Diagnostic Indications
Pericardiocentesis is often performed to assist in the diagnosis of a patient who has accumulation of fluid in the pericardial sac. Pericardiocentesis can help distinguish whether the fluid collection is due to infection, spread of cancer, or possibly an autoimmune condition.[1][2][3]
Therapeutic Indications
- Cardiac tamponade is a condition in which an accumulation of fluid within the pericardium creates excessive pressure, which then prevents the heart from filling normally with blood. This can critically decrease the amount of blood that is pumped from the heart, which can be lethal. The removal of the excess fluid reverses this dangerous process.
- Pericardial effusions larger than 250 mL.
- Pericardial effusions in which size increases despite intensive dialysis for 10-14 days.
Surgery
Pericardiocentesis can be performed for diagnostic purposes and as a therapeutic intervention.[4][5][6][7]
Elective Pericardiocentesis
Preoperative Evaluation
Unless the patient is in extreme and CPR is being performed, a preoperative transthoracic echocardiogram should always be performed to delineate the extent and location of the effusion. At least 1 cm of free-flowing fluid should be accessible from the anterior approach. A chest X ray may be valuable to demonstrate mediastinal shifts, pleural effusions, the position of the diaphragm and the size of the pericardial shadow.
Any abnormal anatomy should be carefully noted. Patients with cancer may have undergone previous surgery and previous radiation therapy and these procedures may shift or distort the normal location of anatomic structures. Patients with lung cancer who have had resection of a lobe may have a shift in the anatomical structures. An enlarged left lobe of the liver extending beyond the mid line may lie in the path of a subxiphoid pericardial needle which carries the risk of a liver laceration. Abdominal distension due to any cause may likewise shift the location of vital structures. If there is concern regarding the patient's anatomy, the procedure should be performed using echocardiographic guidance.
Equipment Needed for Pericardial Drainage
A pericardiocentesis kit should be stocked at all times in a cardiac catheterization laboratory for emergent use. A pericardiocentesis kit should include the following:
- An 18 G bevelled tip or a curved "blunt end" needle (a longer needle such as a lumbar puncture needle may be needed in significantly obese)
- A J tipped 0.035" wire
- A 7F dilator
- A straight or pig tail catheter with side holes
- A properly grounded ECG V lead attached to the needle via an alligator clip. This is used to demonstrate ST elevation upon myocardial contact to prevent entrance of the needle into the right ventricle.
Patient Preparation
A wedge should be placed under the patient's back so that the upper torso is elevated by 30 to 45°. Elevation of the patient's torso minimizes the patient's sense of dyspnea and allows gravitational descent of free flowing fluid to the area of needle entry. It should be noted that a loculated effusion may not collect at the point of entry of the needle. This is again an indication for echocardiographic guidance in the cardiac catheterization laboratory. Also, with the upper torso elevated, the mediastinum assumes a more elongated shape with the diaphragm assuming a more flattened shape further assisting proper direction of the needle. The subxiphoid region should be prepared and draped in the usual sterile fashion. Likewise, the femoral access site should also be prepared and draped in the usual sterile fashion in anticipation of venous and arterial hemodynamic monitoring.
Analgesia and Sedation
Local anesthesia is provided using a 25 G needle to raise a wheal at the puncture site (1-2 mL of 1-2% lidocaine). Deeper infiltration of the subcutaneous tissue is provided with a 22 G needle. Care should be exercised because in a thin patient this needle may in fact enter the pericardial sac. Conscious sedation (according to local practices) can be administered to further reduce patient discomfort and anxiety.
Hemodynamic and Electrocardiographic Monitoring of the Patient
Prior to evacuation of fluid from the pericardium by pericardiocentesis, a right heart catheterization should be performed to demonstrate tamponade physiology (or absence of it) and to monitor the success of pericardial drainage. In patients with tamponade, there will be equalization of the right atrial pressure, the right ventricular end diastolic pressure, and the pulmonary artery end diastolic pressure. In general, the right atrial and pericardial pressures will be elevated to 15 to 20 mm of Hg. Some patients may present with "low pressure tamponade". These patients may be volume dehydrated due to vomiting or other sources of fluid loss. A fluid bolus may assist in clarifying the diagnosis in these patients.
Right atrial pressure and pericardial pressures should be continuously monitored, as these pressures will fall as the fluid is drained. Continuous arterial pressure monitoring with a 4F sheath in the common femoral artery and continuous ECG monitoring should be employed.
The Subxiphoid Approach to Performing Percutaneous Pericardiocentesis
The subxiphoid approach is the most common approach to performing pericardiocentesis. The parasternal and apical approaches should only be attempted with echocardiographic guidance. For the parasternal approach, in order to minimize vascular and pulmonary damage the puncture should be approximately 2 cm to the left of the left sternal border in the 2nd to 4th intercostal spaces and above the corresponding rib. If the needle is directed toward the right shoulder or vertically there is the risk of caval or atrial laceration.
In performing the subxiphoid approach, it is critical that the needle pass below the ribs and sternum. Therefore, to facilitate this approach, considerable pressure should be placed on region just below the sternum to create an area of deep invagination of the epigastrium. Creation of this area of invagination is particularly critical as the needle is inserted. Safe passage of the needle below the ribs will reduce the risk of puncturing or lacerating the subcostal vessels. Considerable pressure may have to be exerted to achieve this. For the subxiphoid approach, a skin incision is made a few millimeters inferior and to the left of the xiphoid process. The needle should be directed to the posterior aspect of the left shoulder. The angle of the needle entering the body is approximately 30 degrees. The goal is to enter the pericardium underlying the right ventricle.
Initially, the needle is advanced with the obturator in place. Once the needle is under the ribs and in the subcutaneous space, the obturator can be removed and a 20 cc syringe is placed on the end of the needle. There are two broad methods to ascertain when the needle has entered the pericardial sac:
- Flashback of pericardial fluid in the 20 cc syringe.
- ST elevation on electrocardiographic ST segment monitoring (optional).
The pericardiocentesis needle can be attached by an alligator clip to a precordial ECG lead, and the ST segments can be monitored continuously. When the needle abuts the right ventricle, ST segment elevation (a current of injury) will be observed. When this is observed the needle should be withdrawn slightly. A three way stopcock can be attached to the needle so that negative pressure can be applied to a 20 cc syringe during needle advancement. Once a flashback of pericardial fluid is obtained, or ST elevation is observed on the precordial lead, the stopcock can then be turned to obtain a hemodynamic tracing to confirm entrance into the pericardial space.
Once the fluid is aspirated the needle is connected to a pressure system via three way stopcock to confirm intra-pericardial physiology and also to rule out its position within a cardiac chamber. A hand contrast injection may be used to confirm the entry in to the pericardial space either through the introducer needle or a 4F dilator. The hematocrit of a frankly bloody effusion is lower than that of the blood.
Before inserting the drainage catheters, insertion of the needle into the pericardial space should be confirmed as follows:
- Hemodynamic monitoring should show that the right atrial (RA) and pericardial pressures are equivalent. A right ventricular pressure waveform should be excluded.
- Injection of 8 cc of dye will demonstrate prolonged swirling appearance of dye in the pericardial cavity rather than the rapid transit of dye out the right ventricular outflow tract.
- If blood is withdrawn, it should not clot and the hematocrit should be lower than that in the systemic circulation. If blood is from the systemic circulation (i.e. the right ventricle), then blood in the syringe will clot.
- Insertion of a 0.035 J tipped wire will not follow the path of the right ventricular outflow tract and will loop in the pericardial sac. The needle can be withdrawn at this point.
A dilator is now advanced in to the pericardial space followed by the drainage catheter which is connected to the three way stopcock. If resistance or kinking in advancing the catheter is encountered, the 0.035” wire can be exchanged to a stiffer wire over the dilator and the attempt repeated. 60 cc of fluid is withdrawn for analysis and the catheter is connected to a negative pressure bottle for drainage. The catheter is then sewn in place and secured.
An echocardiogram can be performed to confirm the drainage. Final pericardial pressure measurements should demonstrate significantly reduced pressure from baseline. If there is persistent elevation of the pressures, then an effusive constrictive pattern of disease may be present.
Echocardiography Guided Pericardiocentesis
Echocardiography may assist in defining the surrounding anatomy and in identifying the exact location of pericardial fluid to facilitate drainage. The method of entry and monitoring of the patient may be identical to the methods mentioned above. However, entry may also be via the parasternal or apical approach, provided adequate echocardiographic windows can be obtained. The needle is advanced under echocardiographic guidance towards the largest accessible fluid collection. Injection of agitated saline may be used to delineate the pericardial space.
Emergency Pericardiocentesis
Portable C Arm Guided Pericardiocentesis
This method can be used in emergencies if neither an echocardiographic guided approach nor a catheterization laboratory are available.
Emergency Pericardiocentesis
Emergency pericardiocentesis may be performed in a patient who has a known pericardial effusion, and who develops profound hemodynamic collapse over the course of minutes rather than hours. This approach may be required in a patient who is found hypotensive and undergoing cardiopulmonary resuscitation on the floor. The approach is the same as described above. Chest compression should be discontinued during advancement of an 18 G needle which is advanced blindly towards the left shoulder. As much fluid as possible should be aspirated. It is notable, however, that the acute accumulation of as little as 50 cc fluid may result in tamponade, and likewise the removal of such a small amount of fluid may reverse the hemodynamic consequences of tamponade.
Pericardial Window
Creation of a pericardial window is a cardiac surgical procedure in which an opening is made in the pericardium to drain fluid that has accumulated around the heart by creating a fistula or "window" from the pericardial space to the peritoneal cavity. Flow of fluid into the peritoneal cavity prevents the accumulation of fluid around the heart (a pericardial effusion), which might cause compression and impaired filling of the heart (cardiac tamponade), a dangerous complication. The procedure is performed for both diagnostic and therapeutic purposes. The creation of a pericardial window is usually performed by a cardiac surgeon or thoracic surgeon who makes an incision, commonly sub-xiphoid, and cuts a small hole in the pericardium. This surgery is performed with local anesthesia. An incision is made either below the sternum, or alternately between the ribs of the left chest. The resection can be with scissors, cautery, a stapling device, or a harmonic scalpel, with no one technique demonstrably better than another. It is best to have a combination of techniques available to resect the pericardium adequately. The surgeon may place a catheter in the pericardial window so that fluid can continue to drain for a short period of time after the surgery. Chest tubes are removed in 2-3 days once the drainage is less than 200cc/24hrs.
Indication
The indication for creation of a pericardial window is a pericardial effusion (fluid build-up) that is either symptomatic, or if the patient is on the verge of cardiac tamponade or if cardiac tamponade has developed. A surgical approach is recommended only in patients with very large chronic effusion, for whom repeated pericardiocentesis have been unsuccessful.
Procedure
Performing a pericardial window traditionally required open-chest surgery, resulting in a large scar and a lengthy recovery time. With the advent of minimally-invasive robotic surgery and the thoracoscopic approach to pericardial window, however, a new approach became available resulting in less trauma, less pain and faster recovery, even that until recently, patients in tamponade were considered unsuitable for a thoracoscopic window.
There are 2 approaches for pericardial window procedure:
1. Subxiphoid approach
A short vertical incision (about 5-7 cm long) is made over the xiphoid process extending onto the midline of the abdomen. The linea alba is incised, and the xiphoid is often completely removed. By finger dissection, the surgeon can reach the retrosternal space. The diaphragmatic aspect of the pericardium is visualized by upward retraction. The pericardium can be grasped with the hook or can be incised directly. A sucker is inserted into the pericardial space and the fluid aspirated. Often a sucker or a finger is used for further dissection of any adhesions.
After all the fluid has been aspirated, the epicardium is inspected and a biopsy specimen can also be taken from the pericardium. A finger is introduced into the pericardial space to determine if any additional adhesions exist or if any nodules are there in the pericardium. Finally, a tube is inserted into the pericardial space and connected through a separate stab wound; the incision is closed in layers.
2. Thoracotomy approach
A small anterior thoracotomy is made in the fourth or fifth intercostal space. To expose the chosen intercostal space, an inframammary skin incision (5-7 cm long) is made, which allows division of the pectoralis muscle. The intercostal space is opened over the superior margin of the rib entering the pleural cavity. A retractor is placed and the pericardium is visualized. The pericardium is incised anterior to the phrenic nerve with a scalpel or scissors. A window is created and the pericardial specimen is sent for histopathology, and samples of pleural effusion are obtained. A chest tube is placed within the pericardium and placed on water seal or suction and the incision is closed in layers.
Complications
If both anterior and posterior pericardial fluid thicknesses measure >1 cm, then the procedure is less likely to be complicated, and the rate of complications is under 2%. Specific complications include the following:[8][9][10][11]
Right Ventricular Puncture
The right ventricle is a very thin walled structure and can be punctured by the needle during pericardiocentesis. Signs of entry into the right ventricular cavity include the following:
- Return of pulsatile blood through the needle.
- The wire will follow the path of the right ventricular outflow tract.
- Hemodynamic monitoring at this point will reveal a right ventricular pressure waveform. It is for this reason that immediately following entrance into what is thought to be the pericardial cavity that hemodynamic monitoring should be initiated. Simultaneous monitoring of the right atrial pressure and the pericardial pressure should indicate that the pressures are equal (usually 15 to 20 mm Hg in the setting of tamponade) and that the pressure waveforms do not have a rapid upstroke to suggest accidental entrance into the right ventricle.
- Injection of 8 cc of dye will demonstrate the rapid transit of dye out the right ventricular outflow tract rather than the prolonged swirling appearance of dye in the pericardial cavity.
- Similar to blood from the systemic circulation, blood in the syringe will clot.
The operator should be certain that the wire is in the pericardial space rather than in the right ventricle before inserting the dilator and drainage tubing as insertion of this larger equipment can create a larger hole in the right ventricle.
Right Atrial Puncture
Right atrial puncture is less well tolerated than right ventricular puncture. It may require surgical repair.
Liver Laceration
Liver laceration ( or intra abdominal visceral trauma) is a rare complication. It occurs more frequently in obese patients and in patients with an enlarged liver (fatty liver). Liver laceration may result in life threatening hemorrhage specially if coagulation is impaired. In intra-abdominal bleeding due to visceral rupture, the cardiac hemodynamics may return to normal transiently, but the patient may then develop features of hemorrhagic shock post procedure.
Coronary Artery Laceration
Coronary artery laceraton is uncommon, especially with the use of a blunt end needle. This complication may be fatal without emergent pericardiocentesis and balloon occlusion of the coronary artery. Emergency pericardiocentesis and surgical repair also may be necessary.
Pneumothorax
A pneumothorax should be suspected if the patient should suddenly become short of breath or hypoxic. The operator should plan quickly under fluoroscopy to make sure that a pneumothorax is not present.
Arrhythmias
Arrhythmias have been reported although sustained life threatening rhythms are rare. A defibrillator should be at the bedside.
Left Ventricular Failure
Left ventricular failure and pulmonary edema can occur in some patients if there is a sudden increase in the loading conditions on the left ventricle with relief of the tamponade physiology.
Other Conditions to Suspect if Hypotension Ensues
If the pericardial sac does not empty adequately and intra-pericardial pressure starts increasing and the patient’s hemodynamic status improves only transiently, then an alternative cause should be sought, such as aortic dissection, laceration of coronary vessels, or free wall rupture.
Autopsy Findings in Patients with Complications
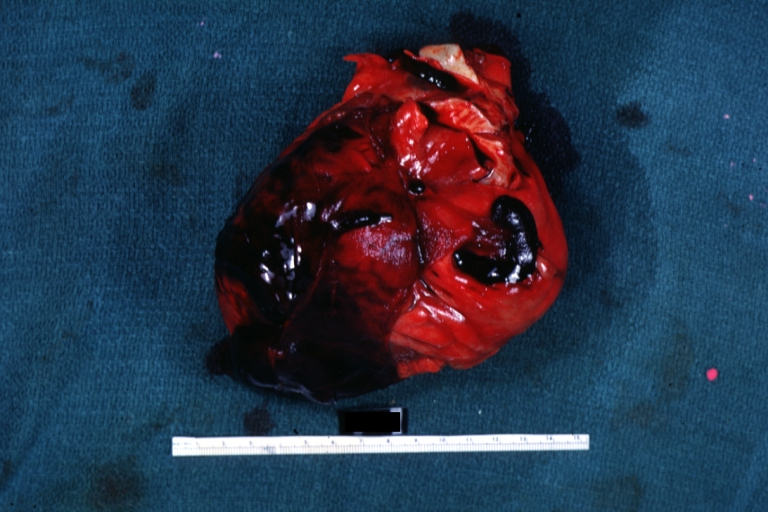
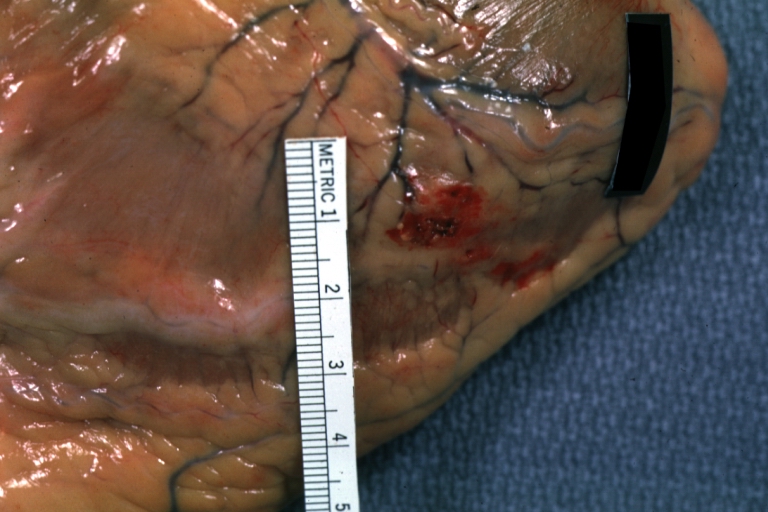
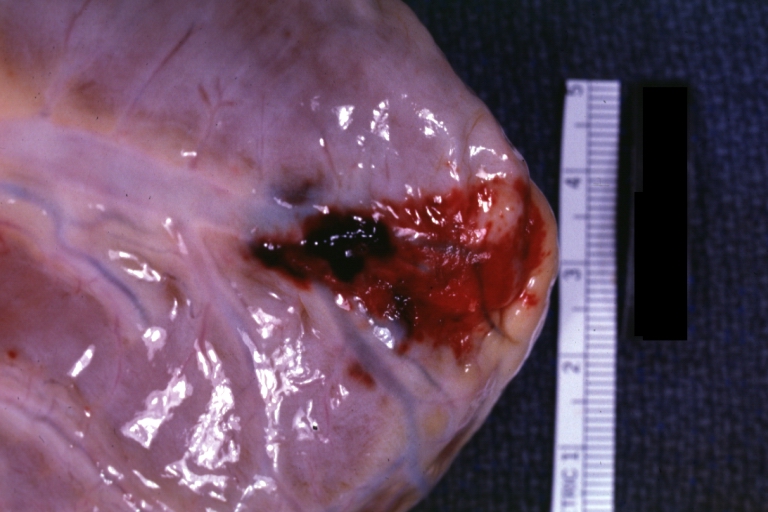
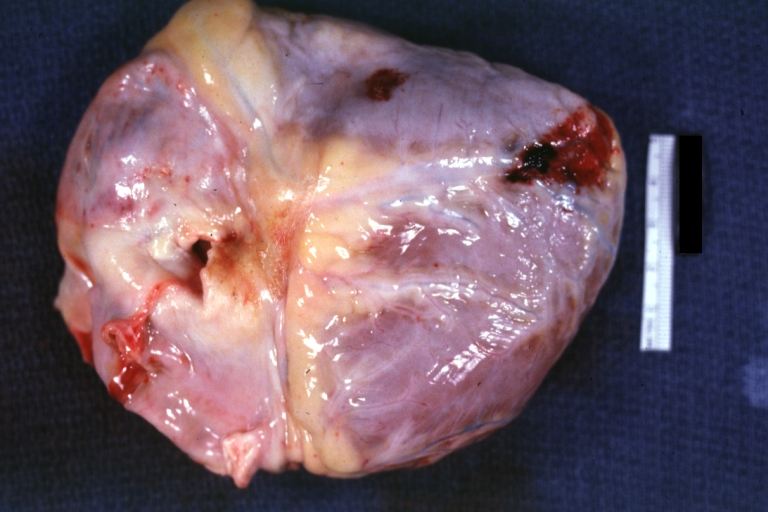

Recovery
Depending on the underlying disease process, patients can be usually discharged in two days.
Post Procedure Care
Antibiotics are not routinely administered. Following relief of pericardial tamponade, some patients may become hypertensive. If drainage stops or slows, the catheter should be repositioned by pulling it back out of the body slightly under suction to redirect to a more favorable position. Catheter occlusion may be prevented by flushing the catheter with either heparinised saline or cath-flo (2 mg of tPA). Once drainage ceases or becomes <100 cc for 24 hrs, the drainage catheter can be removed.
If the right atrial pressure continues to be high with pericardial pressure becoming sub zero then alternative diagnoses such as pericardial constriction, restriction, or elevated right ventricular pressure due to other causes, should be entertained.
2015 ESC Guidelines on the Diagnosis and Treatment of Cardiac tamponade (DO NOT EDIT)[12]
Recommendations for the management of cardiac tamponade
| Class I |
| 1. Urgent pericardiocentesis or cardiac surgery is recommended to treat cardiac tamponade.
2. A judicious clinical evaluation including echocardiographic findings is recommended to guide the timing of pericardiocentesis.(Level of Evidence: C) |
| Class IIb |
| 1. A triage system may be considered to guide the timing of pericardiocentesis.(Level of Evidence: C)
|
| Class III |
| 1. Vasodilators and diuretics are not recommended in the presence of cardiac tamponade.(Level of Evidence: C) |
References
- ↑ Ball JB, Morrison WL (March 1997). "Cardiac tamponade". Postgrad Med J. 73 (857): 141–5. doi:10.1136/pgmj.73.857.141. PMC 2431271. PMID 9135828.
- ↑ Romagano MP, Patel K, Williams S, Apuzzio JJ (2020). "Evaluation and Treatment of Cardiac Tamponade in a Pregnant Patient". Case Rep Obstet Gynecol. 2020: 8703980. doi:10.1155/2020/8703980. PMC 6985926 Check
|pmc=value (help). PMID 32015919 Check|pmid=value (help). - ↑ White JB, Macklin S, Studley JG, Marshall RD (November 1988). "Cardiac tamponade: a review of diagnosis and anaesthetic and surgical management illustrated by three case reports". Ann R Coll Surg Engl. 70 (6): 386–91. PMC 2498622. PMID 3061355.
- ↑ Ramakrishnan S, Marshall AJ, Pickard JG, Tyrrell CJ (August 1988). "Pericardiocentesis and systemic cytotoxic chemotherapy in the management of cardiac tamponade secondary to disseminated breast carcinoma". Br Heart J. 60 (2): 162–4. doi:10.1136/hrt.60.2.162. PMC 1216540. PMID 3415876.
- ↑ Gumrukcuoglu HA, Odabasi D, Akdag S, Ekim H (2011). "Management of Cardiac Tamponade: A Comperative Study between Echo-Guided Pericardiocentesis and Surgery-A Report of 100 Patients". Cardiol Res Pract. 2011: 197838. doi:10.4061/2011/197838. PMC 3177087. PMID 21941665.
- ↑ Haponiuk I, Kwasniak E, Chojnicki M, Jaworski R, Steffens M, Sendrowska A, Gierat-Haponiuk K, Leszczyńska K, Paczkowski K, Zielinski J (April 2015). "Minimally invasive transxiphoid approach for management of pediatric cardiac tamponade - one center's experience". Wideochir Inne Tech Maloinwazyjne. 10 (1): 107–14. doi:10.5114/wiitm.2014.47690. PMC 4414096. PMID 25960801.
- ↑ Adeniran AA, Adegoke OO, Komolafe AO (2018). Pan Afr Med J (in French). 29: 37. doi:10.11604/pamj.2018.29.37.14335. PMC 5987150. PMID 29875919 //www.ncbi.nlm.nih.gov/pmc/articles/PMC5987150. Missing or empty
|title=(help) - ↑ Mujović N, Marinković M, Marković N, Kocijančić A, Kovačević V, Simić D, Ristić A, Stanković G, Miličić B, Putnik S, Vujisić-Tešić B, Potpara TS (October 2016). "Management and Outcome of Periprocedural Cardiac Perforation and Tamponade with Radiofrequency Catheter Ablation of Cardiac Arrhythmias: A Single Medium-Volume Center Experience". Adv Ther. 33 (10): 1782–1796. doi:10.1007/s12325-016-0402-x. PMC 5055551. PMID 27554091.
- ↑ Cypierre A, Pesteil F, Cassat C, Parraf F, Bellier R, Ursulet L, Eveno C, Vignon P, François B (April 2012). "Cardiac tamponade related to a coronary injury by a pericardial calcification: an unusual complication". BMC Cardiovasc Disord. 12: 28. doi:10.1186/1471-2261-12-28. PMC 3353206. PMID 22533452.
- ↑ Fink D, Frigiola A, Cullen S (December 2012). "Postcardiotomy syndrome: recurrent cardiac tamponade and an exquisite steroid response". BMJ Case Rep. 2012. doi:10.1136/bcr-2012-007761. PMC 4544987. PMID 23257941.
- ↑ Goh AC, Lundstrom RJ (October 2015). "Spontaneous Coronary Artery Dissection with Cardiac Tamponade". Tex Heart Inst J. 42 (5): 479–82. doi:10.14503/THIJ-14-4260. PMC 4591893. PMID 26504447.
- ↑ 12.0 12.1 Adler, Yehuda; Charron, Philippe; Imazio, Massimo; Badano, Luigi; Barón-Esquivias, Gonzalo; Bogaert, Jan; Brucato, Antonio; Gueret, Pascal; Klingel, Karin; Lionis, Christos; Maisch, Bernhard; Mayosi, Bongani; Pavie, Alain; Ristić, Arsen D.; Sabaté Tenas, Manel; Seferovic, Petar; Swedberg, Karl; Tomkowski, Witold (2015). "2015 ESC Guidelines for the diagnosis and management of pericardial diseases". European Heart Journal. 36 (42): 2921–2964. doi:10.1093/eurheartj/ehv318. ISSN 0195-668X.