Cardiac amyloidosis pathophysiology
|
Cardiac amyloidosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Cardiac amyloidosis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Cardiac amyloidosis pathophysiology |
|
Risk calculators and risk factors for Cardiac amyloidosis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aarti Narayan, M.B.B.S [2]; Raviteja Guddeti, M.B.B.S. [3]Sabawoon Mirwais, M.B.B.S, M.D.[4]
Overview
The characteristic feature of cardiac amyloidosis is abnormal deposition of abnormally folded light chains of several serum proteins, making them insoluble and leading to their accumulation in various organs. This abnormal folding of proteins is most commonly a result of genetic mutations or excessive formation. Involvement of cardiac muscle can lead to heart failure, arrhythmias, and advanced cardiac conduction disorders.
Pathophysiology
Pathogenesis
- Amyloidosis is characterized by the deposition and extracellular accumulation of fibrillary proteins, leading to the loss of normal tissue architecture.[1]
- Serum proteins are capable of undergoing abnormal beta pleated folding to form amyloid protein.
- The most common culprit proteins responsible for forming amyloid deposits are the light chains of immunoglobulins produced by plasma cells in the bone marrow.[2]
- Cardiac amyloidosis is a disease of the myocardium and it is characterized by extracellular amyloid deposition in the heart.[3]
- The infiltration of the myocardium, resulting in the thickening of ventricular walls, leads to concentric ventricular remodelling and diminished cardiac output.
- The resulting elevated pressure in the atria causes atrial dilatation and the conduction system of the heart can also get affected.[4]
- Intramyocardial vessels can also be infiltrated by the amyloid and this can cause reduced perfusion of the myocardium.[5]
- The most frequent types of amyloidosis involving the heart include:
- Acquired monoclonal immunoglobulin light-chain amyloidosis (Primary amyloidosis)
- Familial or hereditary transthyretin (TTR)-related form
- Systemic senile amyloidosis (Non-mutant TTR-related amyloidosis, Wild-type ATTR)
- All these forms frequently involve the myocardium, with the light chain amyloidosis (AL) being the most common.[6][7]
Acquired Monoclonal Immunoglobulin Light-chain Amyloidosis (AL)
- Monoclonal gammopathy of benign type is the most common form associated with AL cardiac amyloidosis.
- Abnormally folded light chains, produced by defective plasma cells, deposit in tissues resulting in disruption of tissue architecture by causing free radical damage and organ dysfunction.
- Severity of the cardiac dysfunction determines the morbidity and mortality.[8]
- Bone marrow filled with plasma cells by more than 5 - 10% is a poor prognostic indicator.
- The lambda (λ) chains are more likely to be involved in amyloid deposit formation than the kappa (κ) chains.
Hereditary Transthyretin (TTR)-Related Form (ATTR)
- The hereditary or familial form of cardiac amyloidosis is inherited in an autosomal dominant pattern. Mutations leading to substitution of a single amino acid on the protein chain can cause abnormal spacial configuration of the protein, leading to its abnormal deposition in the inter-cellular space.
- The mutations in the genes producing various proteins like transthyretin, fibrinogen, apolipoprotein A1 and A2 are responsible. However, the transthyretin mutation is by far the most common cause of hereditary cardiac amyloidosis.
- Transthyretin, a protein tetramer synthesized in the liver, is responsible for transport of the thyroid hormone and vitamin A in the body. The extent of myocardial involvement varies with the gene sequence mutated in the transthyretin gene.
- About 90 to 100% of patients with "Thr60Ala" mutation, where alanine is substituted for threonine at the 60th position on the transthyretin protein and the "Val122Ile", in which isoleucine is substituted for valine at the 122nd amino acid position, have severe restrictive cardiomyopathy at presentation.
- The "Val30Met" mutation of transthyretin has cardiac involvement only in the elderly patients, with peripheral and autonomic nervous system being the primary target. The Val30Met mutation is normally present in 3.9% of all African Americans and in about 23% of African Americans with cardiac amyloidosis.[9]
- Cardiac involvement is rare in other variant ATTR like mutations in apolipoprotein A1 and secondary amyloidosis. These more often involve the liver and kidneys.
Senile Systemic Amyloidosis (Non-mutant TTR-related amyloidosis, Wild Type ATTR)
- Amyloid deposits are found in the hearts of approximately 25% of elderly patients at autopsy.[9] The clinical significance of these amyloid deposits has not been elucidated, although excess deposition leading to symptoms has been shown to be associated with increased mortality, with a median survival of 7 years following the onset of symptoms.[10][11][12]
- This type of amyloidosis predominantly involves the heart, with the exception of Carpal tunnel syndrome , which presents about 3 to 4 years before cardiac failure.
- This disorder shows male preponderance and commonly presents after 60 years of age. This condition is often misdiagnosed as chronic hypertension, but recent diagnostic techniques like cardiac MRI have made possible the diagnosis of this condition. It is probably the most common form of amyloidosis in the United States.
- Senile systemic amyloidosis has been associated with increased incidence of myocardial infarctions[13] and atrial fibrillation[14] in various studies. The association of tau protein with occurrence of this condition has raised the question of correlation of senile systemic amyloidosis with Alzheimer's disease.
Isolated Atrial Amyloidosis (IAA)
- In isolated atrial amyloid deposition the deposition is limited solely to the atria.
- This condition most commonly results from excess production of abnormally folded atrial natriuretic peptide, like in congestive cardiac failure and valvular heart disease. The amount of amyloid deposited in the atrium correlates with patient's age and P wave duration, which is associated with the magnitude of delay in atrial conduction.[15][16]
- The incidence of this condition increases with age with a female preponderance.[17] IAA first presents in the fourth decade of life, and there is an increased incidence of 15 to 20% per decade of life. More than 90% of the patients with isolated atrial amyloidosis are in their ninth decade of life.[15]
- Increased incidence of atrial fibrillation[18] and other tachyarrhythmias has been noted in patients with isolated atrial amyloidosis.[19]
Other Types of Amyloidosis
- Chronic inflammation causing systemic AA amyloidosis involves the heart tissue in only 2% of the patients, causing heart failure and arrhythmias.
- The incidence of this type of cardiac amyloidosis is progressively decreasing signifying better treatment of rheumatological disorders and chronic infections.
Atrial Fibrillation in Cardiac Amyloidosis
- Autonomic imbalance, degenerative tissue changes, alterations in intercellular matrix and fibrosis increase the likelihood of developing atrial fibrillation in patients with cardiac amyloidosis. Increased amounts of fibrous tissue in the extracellular space disturbs the cell-to-cell coupling, thereby directly affecting conduction.
- In a study done by Leone et al.[20] in patients with chronic persistent AF, amyloid deposits were found mainly in the left atrial appendages. On microscopic analysis, the deposition was predominantly along the sarcolemma of myocytes with minimal involvement of the endocardium.
Genetics
- Cardiac amyloidosis, in the setting of familial or hereditary amyloidosis, can involve mutations in the following genes:[21]
- LYZ gene
- Fibrinogen A alpha polypeptide gene
- FGA gene
- APOA1 gene
- Lysozyme gene
- B2M gene
Associated Conditions
- Conditions associated with AL cardiac amyloidosis include:
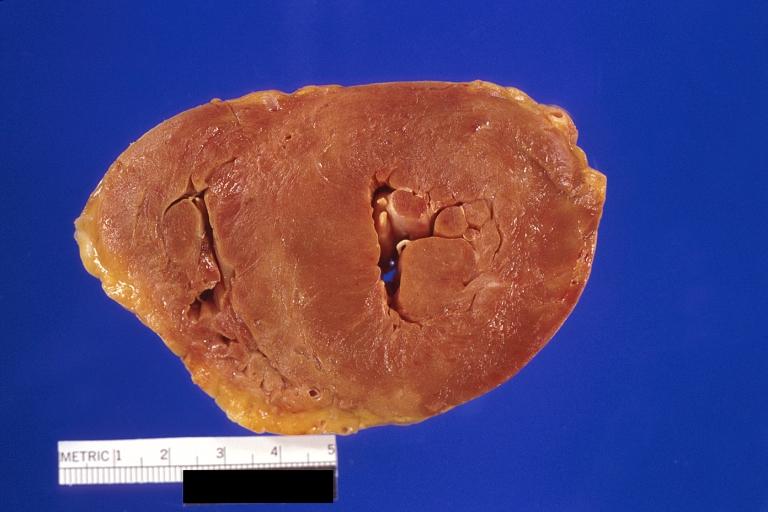
Gross Pathology
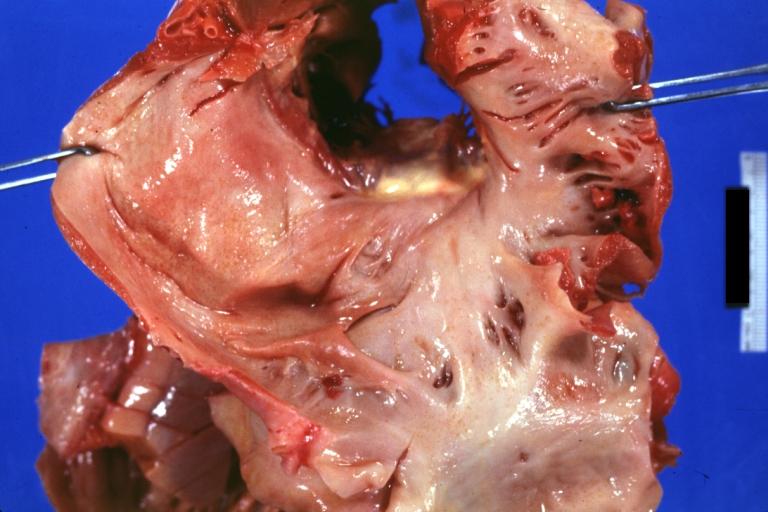
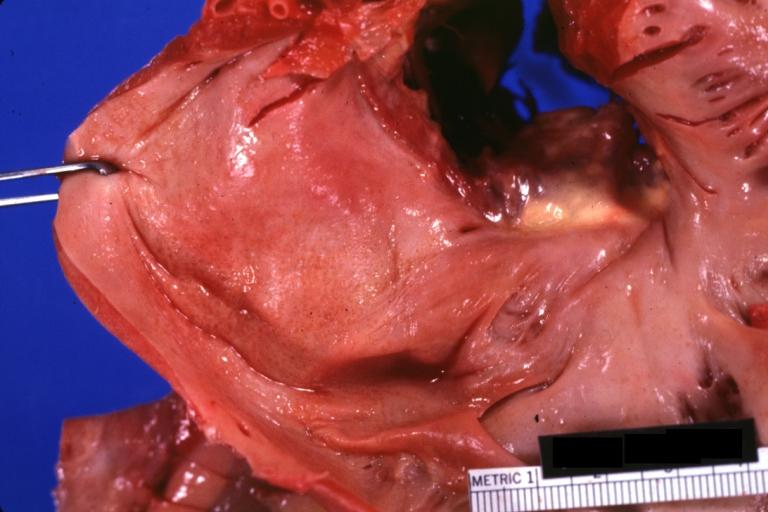
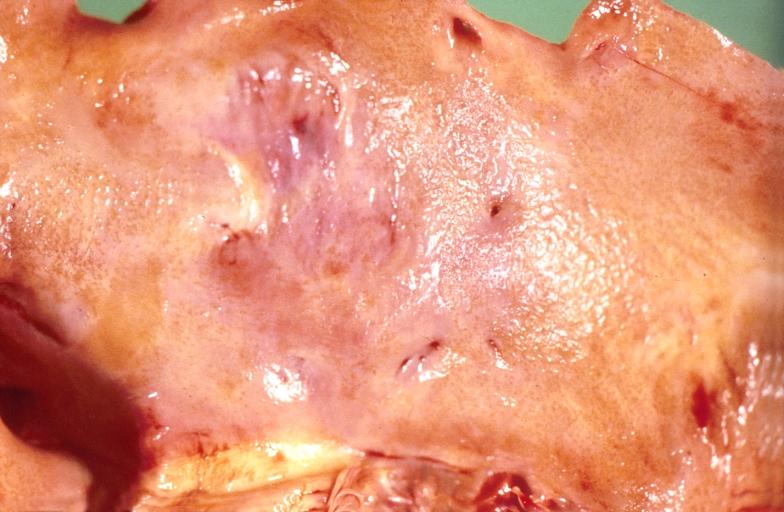
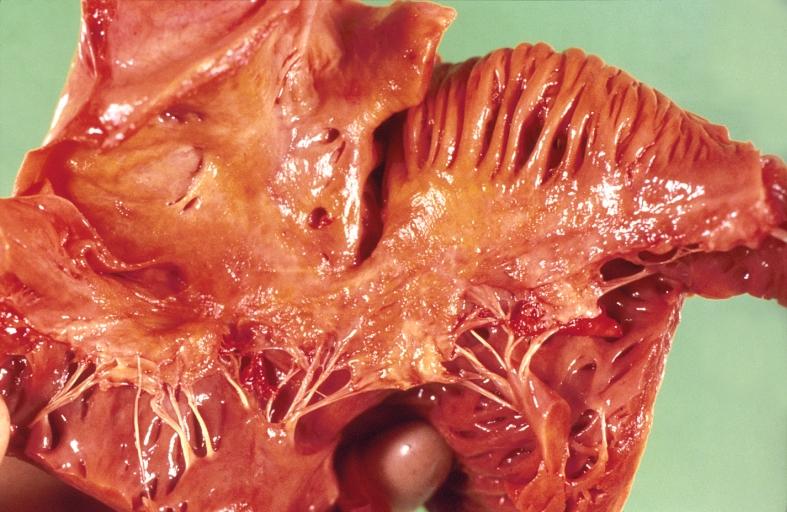
Cardiac amyloid deposits are most commonly seen in the myocardium, but can also be seen in the atria, pericardium, endocardium and microvasculature.
- On gross examination, the myocardium is thicker, firm and rubbery in consistency. More than half of myocardium involvement is common in the AL type of cardiac amyloidosis.
- The size of the ventricular cavity remains unchanged, however filling of the ventricles is restricted (causing restrictive cardiomyopathy) because of stiffening of the ventricular wall as a result of deposition of amyloid material.
- Pericardial effusion and valvular dysfunction is common from pericardial and endocardial involvement. Intracardiac thrombus formation is frequently seen and may result in fatal thromboembolism.[22][23][24]
 |
 |
 |
 |
 |
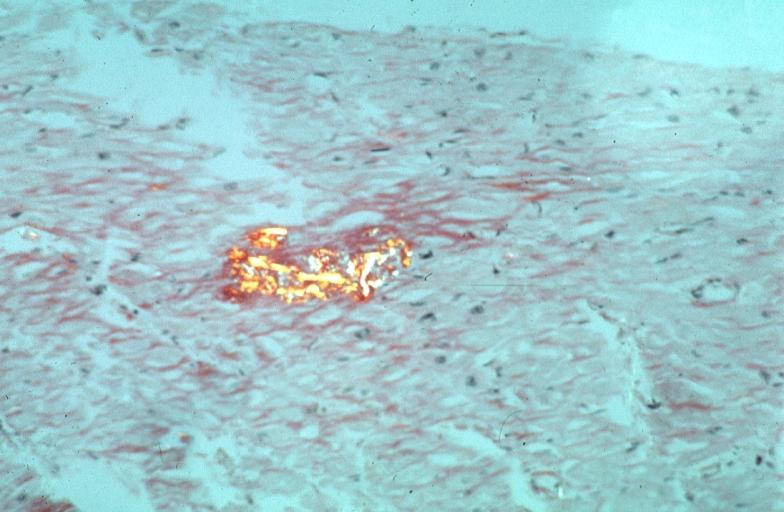
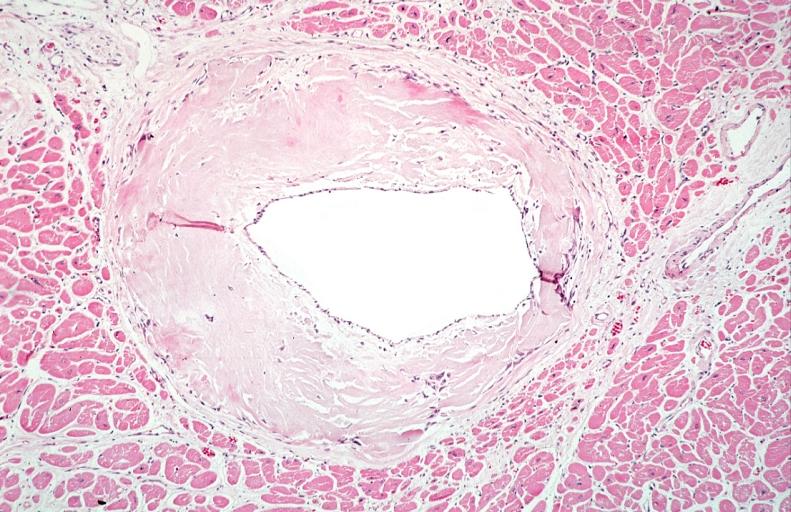
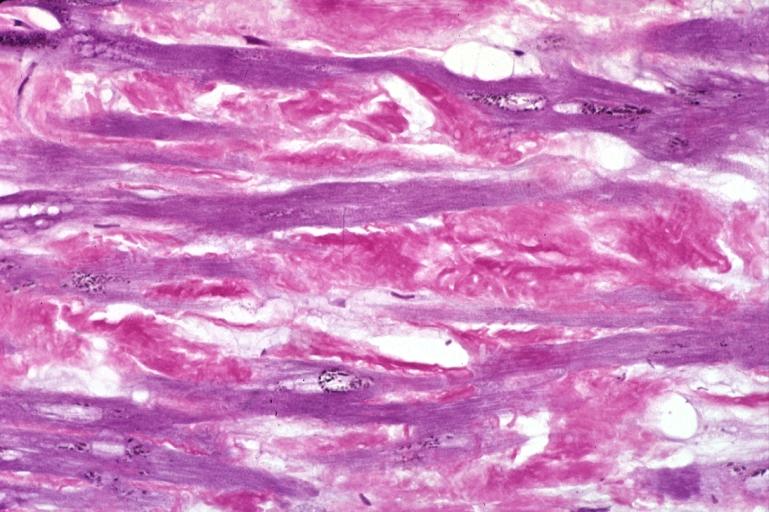
Microscopic Pathology
- Under light microscope, extracellular deposits of hyaline like amyloid material are evident. Resultant myocardial fibrosis restricts the movement of the ventricle, compromising complete filling of the ventricle during diastole.
- Amyloid deposits are also seen in the vasculature, particularly the microvasculature thereby sparing the large epicardial vessels. This leads to myocardial ischemia and tissue infarction.
 |
 |
 |
References
- ↑ Merlini G, Bellotti V (2003). "Molecular mechanisms of amyloidosis". The New England Journal of Medicine. 349 (6): 583–96. doi:10.1056/NEJMra023144. PMID 12904524. Retrieved 2012-02-13. Unknown parameter
|month=ignored (help) - ↑ Martinez-Naharro A, Hawkins PN, Fontana M (April 2018). "Cardiac amyloidosis". Clin Med (Lond). 18 (Suppl 2): s30–s35. doi:10.7861/clinmedicine.18-2-s30. PMC 6334035. PMID 29700090.
- ↑ Martinez-Naharro A, Hawkins PN, Fontana M (April 2018). "Cardiac amyloidosis". Clin Med (Lond). 18 (Suppl 2): s30–s35. doi:10.7861/clinmedicine.18-2-s30. PMC 6334035. PMID 29700090.
- ↑ Dharmarajan K, Maurer MS (2012). "Transthyretin cardiac amyloidoses in older North Americans". Journal of the American Geriatrics Society. 60 (4): 765–74. doi:10.1111/j.1532-5415.2011.03868.x. PMC 3325376. PMID 22329529. Unknown parameter
|month=ignored (help) - ↑ Falk RH (September 2005). "Diagnosis and management of the cardiac amyloidoses". Circulation. 112 (13): 2047–60. doi:10.1161/CIRCULATIONAHA.104.489187. PMID 16186440.
- ↑ Falk RH, Comenzo RL, Skinner M (1997). "The systemic amyloidoses". The New England Journal of Medicine. 337 (13): 898–909. doi:10.1056/NEJM199709253371306. PMID 9302305. Retrieved 2012-02-13. Unknown parameter
|month=ignored (help) - ↑ Gertz MA, Lacy MQ, Dispenzieri A (1999). "Amyloidosis: recognition, confirmation, prognosis, and therapy". Mayo Clinic Proceedings. Mayo Clinic. 74 (5): 490–4. doi:10.4065/74.5.490. PMID 10319082. Retrieved 2012-02-13. Unknown parameter
|month=ignored (help) - ↑ Martinez-Naharro A, Hawkins PN, Fontana M (April 2018). "Cardiac amyloidosis". Clin Med (Lond). 18 (Suppl 2): s30–s35. doi:10.7861/clinmedicine.18-2-s30. PMC 6334035. PMID 29700090.
- ↑ 9.0 9.1 Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD (2012). "Updates in cardiac amyloidosis: a review". Journal of the American Heart Association. 1 (2): e000364. doi:10.1161/JAHA.111.000364. PMC 3487372. PMID 23130126. Unknown parameter
|month=ignored (help) - ↑ Ng B, Connors LH, Davidoff R, Skinner M, Falk RH (2005). "Senile systemic amyloidosis presenting with heart failure: a comparison with light chain-associated amyloidosis". Archives of Internal Medicine. 165 (12): 1425–9. doi:10.1001/archinte.165.12.1425. PMID 15983293. Unknown parameter
|month=ignored (help) - ↑ Hodkinson HM, Pomerance A (1977). "The clinical significance of senile cardiac amyloidosis: a prospective clinico-pathological study". The Quarterly Journal of Medicine. 46 (183): 381–7. PMID 918253. Retrieved 2012-02-13. Unknown parameter
|month=ignored (help) - ↑ Lie JT, Hammond PI (1988). "Pathology of the senescent heart: anatomic observations on 237 autopsy studies of patients 90 to 105 years old". Mayo Clinic Proceedings. Mayo Clinic. 63 (6): 552–64. PMID 3374172. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Tanskanen M, Peuralinna T, Polvikoski T; et al. (2008). "Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study". Annals of Medicine. 40 (3): 232–9. doi:10.1080/07853890701842988. PMID 18382889.
- ↑ Falk RH, Dubrey SW (2010). "Amyloid heart disease". Progress in Cardiovascular Diseases. 52 (4): 347–61. doi:10.1016/j.pcad.2009.11.007. PMID 20109604.
- ↑ 15.0 15.1 Goette A, Röcken C (2004). "Atrial amyloidosis and atrial fibrillation: a gender-dependent "arrhythmogenic substrate"?". European Heart Journal. 25 (14): 1185–6. doi:10.1016/j.ehj.2004.04.014. PMID 15246635. Unknown parameter
|month=ignored (help) - ↑ Millucci L, Ghezzi L, Bernardini G, Braconi D, Tanganelli P, Santucci A (2012). "Prevalence of isolated atrial amyloidosis in young patients affected by congestive heart failure". TheScientificWorldJournal. 2012: 293863. doi:10.1100/2012/293863. PMC 3317626. PMID 22536133.
- ↑ Steiner I (1987). "The prevalence of isolated atrial amyloid". The Journal of Pathology. 153 (4): 395–8. doi:10.1002/path.1711530413. PMID 3430237. Unknown parameter
|month=ignored (help) - ↑ Röcken C, Peters B, Juenemann G; et al. (2002). "Atrial amyloidosis: an arrhythmogenic substrate for persistent atrial fibrillation". Circulation. 106 (16): 2091–7. PMID 12379579. Unknown parameter
|month=ignored (help) - ↑ Ariyarajah V, Steiner I, Hájková P; et al. (2009). "The association of atrial tachyarrhythmias with isolated atrial amyloid disease: preliminary observations in autopsied heart specimens". Cardiology. 113 (2): 132–7. doi:10.1159/000177950. PMID 19039221.
- ↑ Leone O, Boriani G, Chiappini B; et al. (2004). "Amyloid deposition as a cause of atrial remodelling in persistent valvular atrial fibrillation". European Heart Journal. 25 (14): 1237–41. doi:10.1016/j.ehj.2004.04.007. PMID 15246642. Unknown parameter
|month=ignored (help) - ↑ Pepys MB, Hawkins PN, Booth DR, Vigushin DM, Tennent GA, Soutar AK, Totty N, Nguyen O, Blake CC, Terry CJ (April 1993). "Human lysozyme gene mutations cause hereditary systemic amyloidosis". Nature. 362 (6420): 553–7. doi:10.1038/362553a0. PMID 8464497.
- ↑ Nakagawa M, Tojo K, Sekijima Y, Yamazaki KH, Ikeda S (2012). "Arterial thromboembolism in senile systemic amyloidosis: report of two cases". Amyloid : the International Journal of Experimental and Clinical Investigation : the Official Journal of the International Society of Amyloidosis. 19 (2): 118–21. doi:10.3109/13506129.2012.685131. PMID 22583098. Unknown parameter
|month=ignored (help) - ↑ Van de Veire NR, Dierick J, De Sutter J (2012). "Intracardiac emboli as first presentation of cardiac AL amyloidosis". European Heart Journal. 33 (7): 818. doi:10.1093/eurheartj/ehr330. PMC 3345559. PMID 21893485. Unknown parameter
|month=ignored (help) - ↑ Feng D, Edwards WD, Oh JK; et al. (2007). "Intracardiac thrombosis and embolism in patients with cardiac amyloidosis". Circulation. 116 (21): 2420–6. doi:10.1161/CIRCULATIONAHA.107.697763. PMID 17984380. Unknown parameter
|month=ignored (help)