Bicalutamide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Stefano Giannoni [2]; Sree Teja Yelamanchili, MBBS [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Bicalutamide is an antiandrogen that is FDA approved for the treatment of Stage D2 metastatic carcinoma of the prostate in combination with a luteinizing hormone-releasing hormone (LHRH) analog. Common adverse reactions include hot flashes, general pain, back pain, pelvic pain and abdominal pain, asthenia, constipation, infection, nausea, peripheral edema, dyspnea, diarrhea, hematuria, nocturia and anemia..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Stage D2 metastatic carcinoma of the prostate
- One 50 mg tablet once daily (morning or evening)
- In combination with a luteinizing hormone-releasing hormone (LHRH) analog.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of BICALUTAMIDE in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of BICALUTAMIDE in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Bicalutamide FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of BICALUTAMIDE in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of BICALUTAMIDE in pediatric patients.
Contraindications
Hypersensitivity
- Bicalutamide is contraindicated in any patient who has shown a hypersensitivity reaction to the drug or any of the tablet’s components.
- Hypersensitivity reactions including angioneurotic edema and urticaria have been reported.
Women
- Bicalutamide has no indication for women, and should not be used in this population.
Pregnancy
- Bicalutamide may cause fetal harm when administered to a pregnant woman. *Bicalutamide is contraindicated in women, including those who are or may become pregnant.
- There are no studies in pregnant women using bicalutamide.
- If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be appraised of the potential hazard to the fetus.
Warnings
Hepatitis
- Cases of death or hospitalization due to severe liver injury (hepatic failure) have been reported postmarketing in association with the use of bicalutamide.
- Hepatotoxicity in these reports generally occurred within the first three to four months of treatment.
- Hepatitis or marked increases in liver enzymes leading to drug discontinuation occurred in approximately 1% of bicalutamide patients in controlled clinical trials.
- Serum transaminase levels should be measured prior to starting treatment with bicalutamide, at regular intervals for the first four months of treatment, and periodically thereafter.
- If clinical symptoms or signs suggestive of liver dysfunction occur (e.g., nausea, vomiting, abdominal pain, fatigue, anorexia, “flu-like” symptoms, dark urine, jaundice, or right upper quadrant tenderness), the serum transaminases, in particular the serum ALT, should be measured immediately.
- If at any time a patient has jaundice, or their ALT rises above two times the upper limit of normal, bicalutamide should be immediately discontinued with close follow-up of liver function.
Gynecomastia and Breast Pain
- In clinical trials with bicalutamide 150 mg as a single agent for prostate cancer, gynecomastia and breast pain have been reported in up to 38% and 39% of patients, respectively.
Glucose Tolerance
- A reduction in glucose tolerance has been observed in males receiving LHRH agonists.
- This may manifest as diabetes or loss of glycemic control in those with preexisting diabetes.
- Consideration should therefore be given to monitoring blood glucose in patients receiving bicalutamide in combination with LHRH agonists.
Laboratory Tests
- Regular assessments of serum Prostate Specific Antigen (PSA) may be helpful in monitoring the patient’s response.
- If PSA levels rise during bicalutamide therapy, the patient should be evaluated for clinical progression.
- For patients who have objective progression of disease together with an elevated PSA, a treatment-free period of antiandrogen, while continuing the LHRH analogue, may be considered.
Adverse Reactions
Clinical Trials Experience
- In patients with advanced prostate cancer treated with bicalutamide in combination with an LHRH analogue, the most frequent adverse reaction was hot flashes (53%).
- In the multicenter, double-blind, controlled clinical trial comparing bicalutamide 50 mg once daily with flutamide 250 mg three times a day, each in combination with an LHRH analogue, the following adverse reactions with an incidence of 5% or greater, regardless of causality, have been reported.
- Other adverse reactions (greater than or equal to 2%, but less than 5%) reported in the bicalutamide-LHRH analogue treatment group are listed below by body system and are in order of decreasing frequency within each body system regardless of causality.
Body as a Whole
Cardiovascular
- Angina Pectoris
- Congestive Heart Failure
- Myocardial Infarct
- Cardiac Arrest
- Coronary Artery Disorder
- Syncope
Digestive
- Melena
- Rectal Hemorrhage
- Dry Mouth
- Dysphagia
- Gastrointestinal Disorder
- Periodontal Abscess
- Gastrointestinal Carcinoma
Metabolic and Nutritional
Musculoskeletal
- Myalgia
- Leg Cramps
Nervous
Respiratory
Skin and Appendages
- Dry Skin
- Alopecia
- Pruritus
- Herpes Zoster
- Skin Carcinoma
- Skin Disorder
Special Senses
- Cataract specified
Urogenital
- Dysuria
- Urinary Urgency
- Hydronephrosis
- Urinary Tract Disorder
Abnormal Laboratory Test Values:
- Laboratory abnormalities including elevated AST, ALT, bilirubin, BUN, and creatinine and decreased hemoglobin and white cell count have been reported in both bicalutamide-LHRH analog treated and flutamide-LHRH analog treated patients.
Postmarketing Experience
- The following adverse reactions have been identified during postapproval use of bicalutamide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Uncommon cases of hypersensitivity reactions, including angioneurotic edema and urticaria have been seen.
- Cases of interstitial lung disease (some fatal), including interstitial pneumonitis and pulmonary fibrosis, have been reported with bicalutamide. Interstitial lung disease has been reported most often at doses greater than 50 mg.
- A few cases of fatal hepatic failure have been reported.
- Reduction in glucose tolerance, manifesting as diabetes or a loss of glycemic control in those with preexisting diabetes, has been reported during treatment with LHRH agonists.
Drug Interactions
- Clinical studies have not shown any drug interactions between bicalutamide and LHRH analogue (goserelin or leuprolide). There is no evidence that bicalutamide induces hepatic enzymes.
- In vitro studies have shown that R-bicalutamide is an inhibitor of CYP3A4 with lesser inhibitory effects on CYP2C9, 2C19 and 2D6 activity. Clinical studies have shown that with coadministration of bicalutamide, mean midazolam (a CYP3A4 substrate) levels may be increased 1.5 fold (for Cmax) and 1.9 fold (for AUC). Hence, caution should be exercised when bicalutamide is coadministered with CYP3A4 substrates.
- In vitro protein-binding studies have shown that bicalutamide can displace coumarin anticoagulants from binding sites. Prothrombin times should be closely monitored in patients already receiving coumarin anticoagulants who are started on bicalutamide and adjustment of the anticoagulant dose may be necessary.
Use in Specific Populations
Pregnancy
- Based on its mechanism of action, bicalutamide may cause fetal harm when administered to a pregnant woman.
- Bicalutamide is contraindicated in women, including those who are or may become pregnant.
- If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
- While there are no human data on the use of bicalutamide in pregnancy and bicalutamide is not for use in women, it is important to know that maternal use of an androgen receptor inhibitor could affect development of the fetus.
- In animal reproduction studies, male offspring of rats receiving doses of 10 mg/kg/day (approximately 2/3 of clinical exposure at the recommended dose) and above, were observed to have reduced anogenital distance and hypospadias. These pharmacological effects have been observed with other antiandrogens. *No other teratogenic effects were observed in rabbits receiving doses up to 200 mg/kg/day (approximately 1/3 of clinical exposure at the recommended dose) or rats receiving doses up to 250 mg/kg/day (approximately 2 times the clinical exposure at the recommended dose).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Bicalutamide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Bicalutamide during labor and delivery.
Nursing Mothers
- Bicalutamide is not indicated for use in women.
Pediatric Use
- The safety and effectiveness of bicalutamide in pediatric patients have not been established.
- Labeling describing pediatric clinical studies for bicalutamide is approved for AstraZeneca Pharmaceuticals LP’s bicalutamide tablet. However, due to AstraZeneca Pharmaceuticals LP’s marketing exclusivity rights, a description of those clinical studies is not approved for this bicalutamide labeling.
Geriatic Use
- In two studies in patients given 50 or 150 mg daily, no significant relationship between age and steady-state levels of total bicalutamide or the active R-enantiomer has been shown.
Gender
- Bicalutamide has not been studied in women.
Race
There is no FDA guidance on the use of Bicalutamide with respect to specific racial populations.
Renal Impairment
- Renal impairment (as measured by creatinine clearance) had no significant effect on the elimination of total bicalutamide or the active R-enantiomer.
Hepatic Impairment
- Bicalutamide should be used with caution in patients with moderate-to-severe hepatic impairment.
- Bicalutamide is extensively metabolized by the liver.
- Limited data in subjects with severe hepatic impairment suggest that excretion of bicalutamide may be delayed and could lead to further accumulation.
- Periodic liver function tests should be considered for hepatic-impaired patients on long-term therapy.
- No clinically significant difference in the pharmacokinetics of either enantiomer of bicalutamide was noted in patients with mild-to-moderate hepatic disease as compared to healthy controls. However, the half-life of the R-enantiomer was increased approximately 76% (5.9 and 10.4 days for normal and impaired patients, respectively) in patients with severe liver disease (n=4).
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Bicalutamide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Bicalutamide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Monitor serum transaminase levels prior to starting treatment with bicalutamide, at regular intervals for the first four months of treatment and periodically thereafter, and for symptoms or signs suggestive of hepatic dysfunction.
- Consideration should be given to monitoring blood glucose in patients receiving bicalutamide in combination with LHRH agonists.
- Monitoring Prostate Specific Antigen (PSA) is recommended
- Prothrombin times should be closely monitored in patient already receiving coumarin anticoagulants who are started on bicalutamide.
IV Compatibility
There is limited information regarding the compatibility of Bicalutamide and IV administrations.
Overdosage
- Long-term clinical trials have been conducted with dosages up to 200 mg of bicalutamide daily and these dosages have been well tolerated. A single dose of bicalutamide that results in symptoms of an overdose considered to be life threatening has not been established.
There is no specific antidote; treatment of an overdose should be symptomatic.
In the management of an overdose with bicalutamide, vomiting may be induced if the patient is alert. It should be remembered that, in this patient population, multiple drugs may have been taken. Dialysis is not likely to be helpful since bicalutamide is highly protein bound and is extensively metabolized. General supportive care, including frequent monitoring of vital signs and close observation of the patient, is indicated.
Pharmacology
 | |
| Clinical data | |
|---|---|
| Trade names | Casodex |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697047 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | well absorbed |
| Protein binding | 96% |
| Metabolism | hepatic |
| Elimination half-life | 5.8 days |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C18H14F4N2O4S |
| Molar mass | 430.373 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Mechanism of Action
- Bicalutamide is a non-steroidal androgen receptor inhibitor.
- It competitively inhibits the action of androgens by binding to cytosol androgen receptors in the target tissue.
- Prostatic carcinoma is known to be androgen sensitive and responds to treatment that counteracts the effect of androgen and/or removes the source of androgen.
- When bicalutamide is combined with luteinizing hormone releasing hormone (LHRH) analog therapy, the suppression of serum testosterone induced by the LHRH analog is not affected. However, in clinical trials with bicalutamide as a single agent for prostate cancer, rises in serum testosterone and estradiol have been noted.
- In a subset of patients who have been treated with bicalutamide and an LHRH agonist, and who discontinue bicalutamide therapy due to progressive advanced prostate cancer, a reduction in Prostate Specific Antigen (PSA) and/or clinical improvement (antiandrogen withdrawal phenomenon) may be observed.
Structure
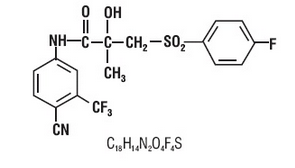
- Bicalutamide tablets contain 50 mg of bicalutamide USP, a non-steroidal androgen receptor inhibitor with no other known endocrine activity. The chemical name is propanamide, N [4 cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methyl-,(+-). The structural and molecular formulas are:
- Bicalutamide has a molecular weight of 430.37. The pKa’ is approximately 12. *Bicalutamide is a fine white to off white powder which is practically insoluble in water at 37°C (5 mg per 1000 mL), slightly soluble in chloroform and absolute ethanol, sparingly soluble in methanol, and soluble in acetone and tetrahydrofuran.
Pharmacodynamics
There is limited information regarding Bicalutamide Pharmacodynamics in the drug label.
Pharmacokinetics
Absorption
- Bicalutamide is well-absorbed following oral administration, although the absolute bioavailability is unknown.
- Coadministration of bicalutamide with food has no clinically significant effect on rate or extent of absorption.
Distribution
- Bicalutamide is highly protein-bound (96%).
Metabolism/Elimination
- Bicalutamide undergoes stereospecific metabolism.
- The S (inactive) isomer is metabolized primarily by glucuronidation. The R (active) isomer also undergoes glucuronidation but is predominantly oxidized to an inactive metabolite followed by glucuronidation.
- Both the parent and metabolite glucuronides are eliminated in the urine and feces.
- The S-enantiomer is rapidly cleared relative to the R-enantiomer, with the R-enantiomer accounting for about 99% of total steady-state plasma levels.
- Pharmacokinetics of the active enantiomer of bicalutamide in normal males and patients with prostate cancer are presented in Table 2.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Two-year oral carcinogenicity studies were conducted in both male and female rats and mice at doses of 5, 15 or 75 mg/kg/day of bicalutamide.
- A variety of tumor target organ effects were identified and were attributed to the antiandrogenicity of bicalutamide, namely, testicular benign interstitial (Leydig) cell tumors in male rats at all dose levels (the steady-state plasma concentration with the 5 mg/kg/day dose is approximately 2/3 human therapeutic concentrations) and uterine adenocarcinoma in female rats at 75 mg/kg/day (approximately 1 1/2 times the human therapeutic concentrations).
- There is no evidence of Leydig cell hyperplasia in patients; uterine tumors are not relevant to the indicated patient population.
- A small increase in the incidence of hepatocellular carcinoma in male mice given 75 mg/kg/day of bicalutamide (approximately 4 times human therapeutic concentrations) and an increased incidence of benign thyroid follicular cell adenomas in rats given 5 mg/kg/day (approximately 2/3 human therapeutic concentrations) and above were recorded.
- These neoplastic changes were progressions of non-neoplastic changes related to hepatic enzyme induction observed in animal toxicity studies.
- Enzyme induction has not been observed following bicalutamide administration in man.
- There were no tumorigenic effects suggestive of genotoxic carcinogenesis.
- A comprehensive battery of both in vitro and in vivo genotoxicity tests (yeast gene conversion, Ames, E. coli, CHO/HGPRT, human lymphocyte cytogenetic, mouse micronucleus, and rat bone marrow cytogenetic tests) has demonstrated that bicalutamide does not have genotoxic activity.
- Administration of bicalutamide may lead to inhibition of spermatogenesis. *The long-term effects of bicalutamide on male fertility have not been studied.
- In male rats dosed at 250 mg/kg/day (approximately 2 times human therapeutic concentrations*), the precoital interval and time to successful mating were increased in the first pairing but no effects on fertility following successful mating were seen.
- These effects were reversed by 7 weeks after the end of an 11-week period of dosing.
- No effects on female rats dosed at 10, 50 and 250 mg/kg/day (approximately 2/3, 1 and 2 times human therapeutic concentrations, respectively) or their female offspring were observed.
- Administration of bicalutamide to pregnant females resulted in feminization of the male offspring leading to hypospadias at all dose levels.
- Affected male offspring were also impotent.
- Based on a maximum dose of 50 mg/day of bicalutamide for an average 70 kg patient.
Clinical Studies
Bicalutamide 50 mg Daily in Combination with an LHRH-A
- In a multicenter, double-blind, controlled clinical trial, 813 patients with previously untreated advanced prostate cancer were randomized to receive bicalutamide 50 mg once daily (404 patients) or flutamide 250 mg (409 patients) three times a day, each in combination with LHRH analogs (either goserelin acetate implant or leuprolide acetate depot).
- In an analysis conducted after a median follow-up of 160 weeks was reached, 213 (52.7%) patients treated with bicalutamide-LHRH analog therapy and 235 (57.5%) patients treated with flutamide-LHRH analog therapy had died.
- There was no significant difference in survival between treatment groups (see Figure 1).
- The hazard ratio for time to death (survival) was 0.87 (95% confidence interval 0.72 to 1.05).

- There was no significant difference in time to objective tumor progression between treatment groups (see Figure 2).
- Objective tumor progression was defined as the appearance of any bone metastases or the worsening of any existing bone metastases on bone scan attributable to metastatic disease, or an increase by 25% or more of any existing measurable extraskeletal metastases.
- The hazard ratio for time to progression of bicalutamide plus LHRH analog to that of flutamide plus LHRH analog was 0.93 (95% confidence interval, 0.79 to 1.1).

- Quality of life was assessed with self-administered patient questionnaires on pain, social functioning, emotional well being, vitality, activity limitation, bed disability, overall health, physical capacity, general symptoms, and treatment related symptoms.
- Assessment of the Quality of Life questionnaires did not indicate consistent significant differences between the two treatment groups.
Safety Data from Clinical Studies using Bicalutamide 150 mg
- Bicalutamide 150 mg is not approved for use either alone or with other treatments.
- Two identical multicenter, randomized, open-label trials comparing bicalutamide150 mg daily monotherapy to castration were conducted in patients that had locally advanced (T3-4, NX, MO) or metastatic (M1) prostate cancer.
Monotherapy — M1 Group
- Bicalutamide150 mg daily is not approved for use in patients with M1 cancer of the prostate.
- Based on an interim analysis of the two trials for survival, the Data Safety Monitoring Board recommended that bicalutamidetreatment be discontinued in the M1 patients because the risk of death was 25% (HR 1.25, 95% CI 0.87 to 1.81) and 31% (HR 1.31, 95% CI 0.97 to 1.77) higher in the bicalutamidetreated group compared to that in the castrated group, respectively.
Locally Advanced (T3-4, NX, MO) Group
- Bicalutamide 150 mg daily is not approved for use in patients with locally advanced (T3-4, NX, M0) cancer of the prostate.
- Following discontinuation of all M1 patients, the trials continued with the T3-4, NX, MO patients until study completion.
- In the larger trial (N=352), the risk of death was 25% (HR 1.25, 95% CI 0.92 to 1.71) higher in the bicalutamide group and in the smaller trial (N=140), the risk of death was 36% (HR 0.64, 95% CI, 0.39 to 1.03) lower in the bicalutamide group.
- In addition to the above two studies, there are three other on-going clinical studies that provide additional safety information for bicalutamide 150 mg, a dose that is not approved for use.
- These are three multicenter, randomized, double-blind, parallel group trials comparing bicalutamide 150 mg daily monotherapy (adjuvant to previous therapy or under watchful waiting) with placebo, for death or time to disease progression, in a population of 8113 patients with localized or locally advanced prostate cancer.
- Bicalutamide150 mg daily is not approved for use as therapy for patients with localized prostate cancer who are candidates for watchful waiting.
- Data from a planned subgroup analysis of two of these trials in 1627 patients with localized prostate cancer who were under watchful waiting, revealed a trend toward decreased survival in the bicalutamide arm after a median follow-up of 7.4 years.
- There were 294 (37.7%) deaths in the bicalutamide treated patients versus 279 (32.9%) deaths in the placebo treated patients (localized watchful waiting group) for a hazard ratio of 1.16 (95% CI 0.99 to 1.37).
How Supplied
- White to off white, circular, biconvex, film-coated tablets debossed with “485” on one side and plain on other side.
- Bottles of 30’s with Child Resistant Cap……….…..…. NDC 47335-485-83
- Bottles of 100’s with Child Resistant Cap………….…..NDC 47335-485-88
- Bottles of 100’s with Non Child Resistant Cap…..…….NDC 47335-485-08
- Bottles of 1000’s with Non Child Resistant Cap……….NDC 47335-485-18
Storage
- Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C (59° and 86°F)
Images
Drug Images
{{#ask: Page Name::Bicalutamide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Bicalutamide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be informed that therapy with bicalutamide tabletsand the LHRH analog should be started at the same time and that they should not interrupt or stop taking these medications without consulting their physician.
- During treatment with bicalutamide tablets, somnolence has been reported, and those patients who experience this symptom should observe caution when driving or operating machines.
- Patients should be informed that diabetes, or loss of glycemic control in patients with preexisting diabetes has been reported during treatment with LHRH agonists.
- Consideration should therefore be given to monitoring blood glucose in patients receiving bicalutamide tablets in combination with LHRH agonists.
Precautions with Alcohol
Alcohol-BICALUTAMIDE interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Casodex[1]
Look-Alike Drug Names
There is limited information regarding Bicalutamide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Bicalutamide |Pill Name=CASODEX_NDC_03100705.jpg |Drug Name=CASODEX |Pill Ingred=BICALUTAMIDE[BICALUTAMIDE]|+sep=; |Pill Imprint=CDX50 |Pill Dosage=50 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=7 |Pill Scoring=1 |Pill Image= |Drug Author=AstraZeneca Pharmaceuticals LP |NDC=03100705
}}
{{#subobject:
|Page Name=Bicalutamide |Pill Name=Bicalutamide_NDC_03787017.jpg |Drug Name=Bicalutamide |Pill Ingred=Bicalutamide[Bicalutamide]|+sep=; |Pill Imprint=M;C17 |Pill Dosage=50 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=6 |Pill Scoring=1 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc |NDC=03787017
}}
{{#subobject:
|Page Name=Bicalutamide |Pill Name=Bicalutamide_NDC_519910560.jpg |Drug Name=Bicalutamide |Pill Ingred=Bicalutamide[Bicalutamide]|+sep=; |Pill Imprint=BCM;50 |Pill Dosage=50 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=7 |Pill Scoring=1 |Pill Image= |Drug Author=Breckenridge Pharmaceutical, Inc. |NDC=519910560
}}
{{#subobject:
|Page Name=Bicalutamide |Pill Name=Bicalutamide_NDC_604290226.jpg |Drug Name=Bicalutamide |Pill Ingred=Bicalutamide[Bicalutamide]|+sep=; |Pill Imprint=BCM;50 |Pill Dosage=50 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=7 |Pill Scoring=1 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290226
}}
{{#subobject:
|Page Name=Bicalutamide |Pill Name=Bicalutamide_NDC_00930220.jpg |Drug Name=Bicalutamide |Pill Ingred=BICALUTAMIDE[BICALUTAMIDE]|+sep=; |Pill Imprint=93;220 |Pill Dosage=50 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=7 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00930220
}}
{{#subobject:
|Page Name=Bicalutamide |Pill Name=Bicalutamide_NDC_167140571.jpg |Drug Name=Bicalutamide |Pill Ingred=BICALUTAMIDE[BICALUTAMIDE]|+sep=; |Pill Imprint=B;50 |Pill Dosage=50 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=7 |Pill Scoring=1 |Pill Image= |Drug Author=Northstar Rx LLC |NDC=167140571
}}
{{#subobject:
|Label Page=Bicalutamide |Label Name=Bicalutamide Package.png
}}
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed KEGG identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugboxes which contain changes to verified fields
- Drug
- Chemotherapeutic agents