Prostate cancer
For patient information click here
Template:DiseaseDisorder infobox
|
Prostate cancer Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Prostate cancer On the Web |
|
American Roentgen Ray Society Images of Prostate cancer |
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Symptoms
Pathophysiology
Causes
Treatment
Medical therapy | Surgical options | Metastasis Treatment | Primary prevention | Secondary prevention | Financial costs | Future therapies
Screening
Diagnosis
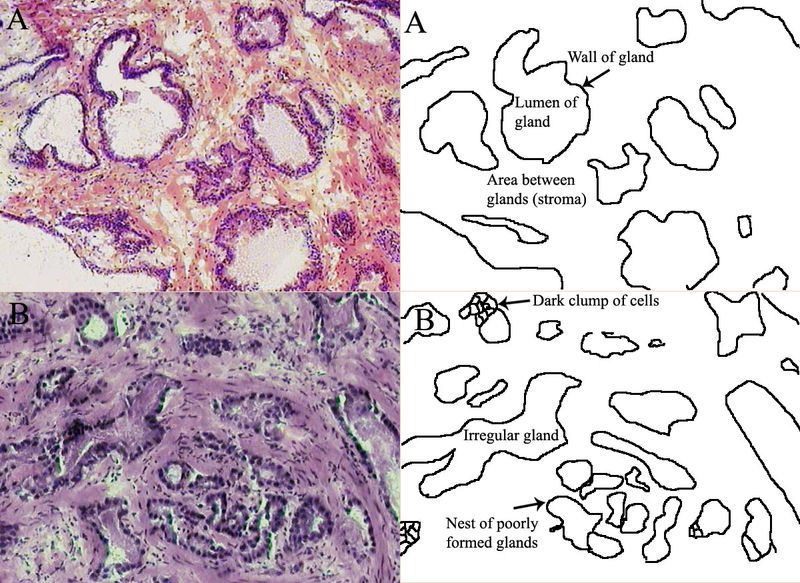
When a man has symptoms of prostate cancer, or a screening test indicates an increased risk for cancer, more invasive evaluation is offered.
The only test which can fully confirm the diagnosis of prostate cancer is a biopsy, the removal of small pieces of the prostate for microscopic examination. However, prior to a biopsy, several other tools may be used to gather more information about the prostate and the urinary tract. Cystoscopy shows the urinary tract from inside the bladder, using a thin, flexible camera tube inserted down the urethra. Transrectal ultrasonography creates a picture of the prostate using sound waves from a probe in the rectum.
- History and Symptoms | Physical Examination | Staging | Lab Studies | Electrocardiogram | X Ray | MRI | CT | Echocardiography | Other imaging findings | Other diagnostic studies
Staging
An important part of evaluating prostate cancer is determining the stage, or how far the cancer has spread. Knowing the stage helps define prognosis and is useful when selecting therapies. The most common system is the four-stage TNM system (abbreviated from Tumor/Nodes/Metastases). Its components include the size of the tumor, the number of involved lymph nodes, and the presence of any other metastases.
The most important distinction made by any staging system is whether or not the cancer is still confined to the prostate. In the TNM system, clinical T1 and T2 cancers are found only in the prostate, while T3 and T4 cancers have spread elsewhere. Several tests can be used to look for evidence of spread. These include computed tomography to evaluate spread within the pelvis, bone scans to look for spread to the bones, and endorectal coil magnetic resonance imaging to closely evaluate the prostatic capsule and the seminal vesicles. Bone scans should reveal osteoblastic appearance due to increased bone density in the areas of bone metastasis - opposite to what is found in many other cancers that metastasize.
Computed tomography (CT) and magnetic resonance imaging (MRI) currently do not add any significant information in the assessment of possible lymph node metastases in patients with prostate cancer according to a meta-analysis.[1] The sensitivity of CT was 42% and specificity of CT was 82%. The sensitivity of MRI was 39% and the specificity of MRI was 82%. For patients at similar risk to those in this study (17% had positive pelvic lymph nodes in the CT studies and 30% had positive pelvic lymph nodes in the MRI studies), this leads to a positive predictive value (PPV) of 32.3% with CT, 48.1% with MRI, and negative predictive value (NPV) of 87.3% with CT, 75.8% with MRI.
After a prostate biopsy, a pathologist looks at the samples under a microscope. If cancer is present, the pathologist reports the grade of the tumor. The grade tells how much the tumor tissue differs from normal prostate tissue and suggests how fast the tumor is likely to grow. The Gleason system is used to grade prostate tumors from 2 to 10, where a Gleason score of 10 indicates the most abnormalities. The pathologist assigns a number from 1 to 5 for the most common pattern observed under the microscope, then does the same for the second most common pattern. The sum of these two numbers is the Gleason score. The Whitmore-Jewett stage is another method sometimes used. Proper grading of the tumor is critical, since the grade of the tumor is one of the major factors used to determine the treatment recommendation.
Risk assessment
Many prostate cancers are not destined to be lethal, and most men will ultimately die from causes other than of the disease. Decisions about treatment type and timing may therefore be informed by an estimation of the risk that the tumor will ultimately recur after treatment and/or progress to metastases and mortality. Several tools are available to help predict outcomes such as pathologic stage and recurrence after surgery or radiation therapy. Most combine stage, grade, and PSA level, and some also add the number or percent of biopsy cores positive, age, and/or other information.
The D’Amico classification stratifies men to low, intermediate, or high risk based on stage, grade, and PSA. It is used widely in clinical practice and research settings. The major downside to the 3-level system is that it does not account for multiple adverse parameters (e.g., high Gleason score and high PSA) in stratifying patients.
The Partin tables predict pathologic outcomes (margin status, extraprostatic extension, and seminal vesicle invasion) based on the same 3 variables, and are published as lookup tables.
The Kattan nomograms predict recurrence after surgery and/or radiation therapy, based on data available either at time of diagnosis or after surgery. The nomograms can be calculated using paper graphs, or using software available on a website or for handheld computers. The Kattan score represents the likelihood of remaining free of disease at a given time interval following treatment.
The UCSF Cancer of the Prostate Risk Assessment (CAPRA) score predicts both pathologic status and recurrence after surgery. It offers comparable accuracy as the Kattan preoperative nomogram, and can be calculated without paper tables or a calculator. Points are assigned based on PSA, Grade, stage, age, and percent of cores positive; the sum yields a 0–10 score, with every 2 points representing roughly a doubling of risk of recurrence. The CAPRA score was derived from community-based data in the CaPSURE database. It has been validated among over 10,000 prostatectomy patients, including patients from CaPSURE; the SEARCH registry, representing data from several Veterans Administration and active military medical centers; a multi-institutional cohort in Germany; and the prostatectomy cohort at Johns Hopkins University.
Treatment
Treatment for prostate cancer may involve watchful waiting, surgery, radiation therapy including brachytherapy (prostate brachytherapy) and external beam radiation, High Intensity Focused Ultrasound (HIFU), chemotherapy, cryosurgery, hormonal therapy, or some combination. Which option is best depends on the stage of the disease, the Gleason score, and the PSA level. Other important factors are the man's age, his general health, and his feelings about potential treatments and their possible side effects. Because all treatments can have significant side effects, such as erectile dysfunction and urinary incontinence, treatment discussions often focus on balancing the goals of therapy with the risks of lifestyle alterations.
The selection of treatment options may be a complex decision involving many factors. For example, radical prostatectomy after primary radiation failure is a very technically challenging surgery and may not be an option.[2] This may enter into the treatment decision.
If the cancer has spread beyond the prostate, treatment options significantly change, so most doctors who treat prostate cancer use a variety of nomograms to predict the probability of spread. Treatment by watchful waiting, HIFU, radiation therapy, cryosurgery, and surgery are generally offered to men whose cancer remains within the prostate. Hormonal therapy and chemotherapy are often reserved for disease which has spread beyond the prostate. However, there are exceptions: radiation therapy may be used for some advanced tumors, and hormonal therapy is used for some early stage tumors. Cryotherapy, hormonal therapy, and chemotherapy may also be offered if initial treatment fails and the cancer progresses.
Active Surveillance
Active Surveillance refers to observation and regular monitoring without invasive treatment. Active surveillance is often used when an early stage, slow-growing prostate cancer is found in an older man. Conversely watchful waiting may also be suggested when the risks of surgery, radiation therapy, or hormonal therapy outweigh the possible benefits. Other treatments can be started if symptoms develop, or if there are signs that the cancer growth is accelerating (e.g., rapidly rising PSA, increase in Gleason score on repeat biopsy, etc.). Most men who choose active surveillance for early stage tumors eventually have signs of tumor progression, and they may need to begin treatment within three years.[3] Although men who choose active surveillance avoid the risks of surgery and radiation, the risk of metastasis (spread of the cancer) may be increased.
For younger men, a trial of active surveillance may not mean avoiding treatment altogether, but may reasonably allow a delay of a few years or more, during which time the quality of life impact of active treatment can be avoided. Published data to date suggest that carefully selected men will not miss a window for cure with this approach. Additional health problems that develop with advancing age during the observation period can also make it harder to undergo surgery and radiation therapy.
Natural Therapy
As an alternative to active surveillance or invasive treatments, which does nothing to change the course of disease, a growing number of clinicians and researchers are looking at non-invasive ways to help men with apparently localized prostate cancer. Perhaps most convincing among this group are Dean Ornish, MD and colleagues, previously made famous for showing that aggressive lifestyle changes can reverse atherosclerosis, and now showing that PSA can be lowered in men with apparent localized prostate cancer using a vegan diet (fish allowed), regular exercise, and stress reduction.[4] These results have so far proven durable after two-years' treatment.[5]
Many other single agents have been shown to reduce PSA, slow PSA doubling times, or have similar effects on secondary markers in men with localized cancer in short term trials, such as the Wonderful variety of pomegranate juice 8 oz daily or genistein, an isoflavone found in various legumes, 60 mg per day.[6][7] The potential of using multiple such agents in concert, let alone combining them with lifestyle changes, has not yet been studied but the potential is great. This is particularly true because most of these natural approaches have very low adverse effect rates, and in fact tend to help other risk factors and disease conditions such as atherosclerosis, diabetes, and risk for other cancers at the same time they are helping slow down prostate cancer. A more thorough review of natural approaches to prostate cancer has been published.[8]
Surgery
Surgical removal of the prostate, or prostatectomy, is a common treatment either for early stage prostate cancer, or for cancer which has failed to respond to radiation therapy. The most common type is radical retropubic prostatectomy, when the surgeon removes the prostate through an abdominal incision. Another type is radical perineal prostatectomy, when the surgeon removes the prostate through an incision in the perineum, the skin between the scrotum and anus. Radical prostatectomy can also be performed laparoscopically, through a series of small (1cm) incisions in the abdomen, with or without the assistance of a surgical robot.
Radical prostatectomy is effective for tumors which have not spread beyond the prostate;[9] cure rates depend on risk factors such as PSA level and Gleason grade. However, it may cause nerve damage that significantly alters the quality of life of the prostate cancer survivor.
Radical prostatectomy has traditionally been used alone when the cancer is small. In the event of positive margins or locally advanced disease found on pathology, adjuvant radiation therapy may offer improved survival. Surgery may also be offered when a cancer is not responding to radiation therapy. However, because radiation therapy causes tissue changes, prostatectomy after radiation has a higher risk of complications.
Laparoscopic radical prostatectomy, LRP, is a new way to approach the prostate surgically with intent to cure. Contrasted with the open surgical form of prostate cancer surgery, laparoscopic radical prostatectomy does not require a large incision. Relying on modern technology, such as miniaturization, fiber optics, and the like, laparoscopic radical prostatectomy is a minimally invasive prostate cancer treatment.
In the hands of an experienced surgeon, robotic assisted laparoscopic prostatectomy (RALP) may reduce positive surgical margins when compared to radical retropubic prostatectomy (RRP) among patients with prostate cancer according to a retrospective study.[1] The relative risk reduction was 57.7%. For patients at similar risk to those in this study (35.5% of patients had positive surgical margins following RRP), this leads to an absolute risk reduction of 20.5%. 4.9 patients must be treated for one to benefit (number needed to treat = 4.9). The relative merits of RALP and benefits over open radical prostatectomy are an area of intense research currently in urology and no definitive data, that has been widely accepted by the broader urological community, exists to say it is superior to a open radical retropubic prostatectomy.
Transurethral resection of the prostate, commonly called a "TURP," is a surgical procedure performed when the tube from the bladder to the penis (urethra) is blocked by prostate enlargement. TURP is generally for benign disease and is not meant as definitive treatment for prostate cancer. During a TURP, a small instrument (cystoscope) is placed into the penis and the blocking prostate is cut away.
In metastatic disease, where cancer has spread beyond the prostate, removal of the testicles (called orchiectomy) may be done to decrease testosterone levels and control cancer growth. (See hormonal therapy, below).
The most common serious complications of surgery are loss of urinary control and impotence. Reported rates of both complications vary widely depending on how they are assessed, by whom, and how long after surgery, as well as the setting (e.g., academic series vs. community-based or population-based data). Although penile sensation and the ability to achieve orgasm usually remain intact, erection and ejaculation are often impaired. Medications such as sildenafil (Viagra), tadalafil (Cialis), or vardenafil (Levitra) may restore some degree of potency. For most men with organ-confined disease, a more limited "nerve-sparing" technique may help reduce urinary incontinence and impotence.[10]
Radiation therapy
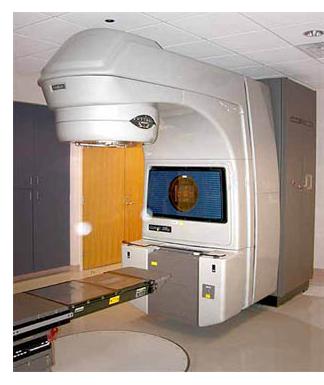
Radiation therapy, also known as radiotherapy, is often used to treat all stages of prostate cancer, or when surgery fails. Radiotherapy uses ionizing radiation to kill prostate cancer cells. When absorbed in tissue, Ionizing radiation such as Gamma and x-rays damage the DNA in cells, which increases the probability of apoptosis (cell death). Two different kinds of radiation therapy are used in prostate cancer treatment: external beam radiation therapy and brachytherapy (specifically prostate brachytherapy).
External beam radiation therapy uses a linear accelerator to produce high-energy x-rays which are directed in a beam towards the prostate. A technique called Intensity Modulated Radiation Therapy (IMRT) may be used to adjust the radiation beam to conform with the shape of the tumor, allowing higher doses to be given to the prostate and seminal vesicles with less damage to the bladder and rectum. External beam radiation therapy is generally given over several weeks, with daily visits to a radiation therapy center. New types of radiation therapy may have fewer side effects than traditional treatment. One of these is Tomotherapy.
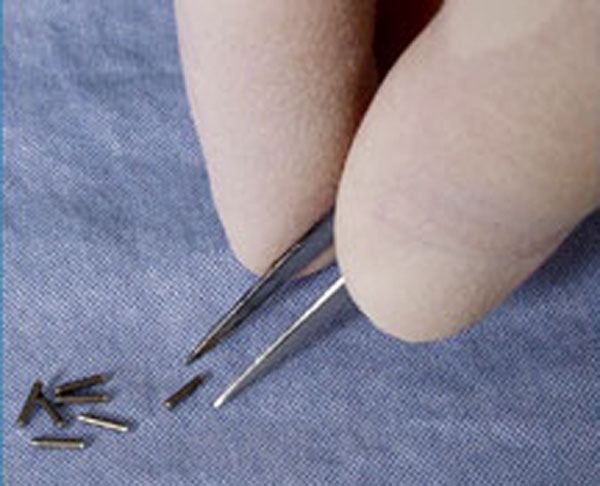
Permanent implant brachytherapy is a popular treatment choice for patients with low to intermediate risk features, can be performed on an outpatient basis, and is associated with good 10-year outcomes with relatively low morbidity[11] It involves the placement of about 100 small "seeds" containing radioactive material (such as iodine-125 or palladium-103) with a needle through the skin of the perineum directly into the tumor while under spinal or general anesthetic. These seeds emit lower-energy X-rays which are only able to travel a short distance. Although the seeds eventually become inert, they remain in the prostate permanently. The risk of exposure to others from men with implanted seeds is generally accepted to be insignificant.[12]
Radiation therapy is commonly used in prostate cancer treatment. It may be used instead of surgery or after surgery in early stage prostate cancer. In advanced stages of prostate cancer radiation is used to treat painful bone metastases. Radiation treatments also can be combined with hormonal therapy for intermediate risk disease, when radiation therapy alone is less likely to cure the cancer. Some radiation oncologists combine external beam radiation and brachytherapy for intermediate to high risk situations. One study found that the combination of six months of androgen suppressive therapy combined with external beam radiation had improved survival compared to radiation alone in patients with localized prostate cancer.[13] Others use a "triple modality" combination of external beam radiation therapy, brachytherapy, and hormonal therapy.
Radiation therapy uses high-energy rays or particles to kill cancer cells.[14] When delivered in the correct dosage, radiation can reduce the risk of recurrence.
Less common applications for radiotherapy are when cancer is compressing the spinal cord, or sometimes after surgery, such as when cancer is found in the seminal vesicles, in the lymph nodes, outside the prostate capsule, or at the margins of the biopsy.
Radiation therapy is often offered to men whose medical problems make surgery more risky. Radiation therapy appears to cure small tumors that are confined to the prostate just about as well as surgery. However, some issues remain unresolved, such as whether radiation should be given to the rest of the pelvis, how much the absorbed dose should be, and whether hormonal therapy should be given at the same time.
Side effects of radiation therapy might occur after a few weeks into treatment. Both types of radiation therapy may cause diarrhea and mild rectal bleeding due to radiation proctitis, as well as urinary incontinence and impotence. Symptoms tend to improve over time. Rates for impotence when comparing radiation to nerve-sparing surgery are similar. Radiation has lower rates of incontinence but higher rates of occasional mild rectal bleeding.[15] Men who have undergone external beam radiation therapy may have a slightly higher risk of later developing colon cancer and bladder cancer.[16]
Cryosurgery
Cryosurgery is another method of treating prostate cancer in which the prostate gland is exposed to freezing temperatures.[17] It is less invasive than radical prostatectomy, and general anesthesia is less commonly used. Under ultrasound guidance, a method invented by Dr. Gary Onik,[18] metal rods are inserted through the skin of the perineum into the prostate. Highly purified Argon gas is used to cool the rods, freezing the surrounding tissue at −186 °C (−302 °F). As the water within the prostate cells freeze, the cells die. The urethra is protected from freezing by a catheter filled with warm liquid. Cryosurgery generally causes fewer problems with urinary control than other treatments, but impotence occurs up to ninety percent of the time. When used as the initial treatment for prostate cancer and in the hands of an experienced cryosurgeon, cryosurgery has a 10 year biochemical disease free rate superior to all other treatments including radical prostatectomy and any form of radiation.[19] Cryosurgery has also been demonstrated to be superior to radical prostatectomy for recurrent cancer following radiation therapy.
Hormonal therapy

Hormonal therapy uses medications or surgery to block prostate cancer cells from getting dihydrotestosterone (DHT), a hormone produced in the prostate and required for the growth and spread of most prostate cancer cells. Blocking DHT often causes prostate cancer to stop growing and even shrink. However, hormonal therapy rarely cures prostate cancer because cancers which initially respond to hormonal therapy typically become resistant after one to two years. Hormonal therapy is therefore usually used when cancer has spread from the prostate. It may also be given to certain men undergoing radiation therapy or surgery to help prevent return of their cancer.[20]
Hormonal therapy for prostate cancer targets the pathways the body uses to produce DHT. A feedback loop involving the testicles, the hypothalamus, and the pituitary, adrenal, and prostate glands controls the blood levels of DHT. First, low blood levels of DHT stimulate the hypothalamus to produce gonadotropin releasing hormone (GnRH). GnRH then stimulates the pituitary gland to produce luteinizing hormone (LH), and LH stimulates the testicles to produce testosterone. Finally, testosterone from the testicles and dehydroepiandrosterone from the adrenal glands stimulate the prostate to produce more DHT. Hormonal therapy can decrease levels of DHT by interrupting this pathway at any point. There are several forms of hormonal therapy:
- Orchiectomy is surgery to remove the testicles. Because the testicles make most of the body's testosterone, after orchiectomy testosterone levels drop. Now the prostate not only lacks the testosterone stimulus to produce DHT, but also it does not have enough testosterone to transform into DHT.
- Antiandrogens are medications such as flutamide, bicalutamide, nilutamide, and cyproterone acetate which directly block the actions of testosterone and DHT within prostate cancer cells.
- Medications which block the production of adrenal androgens such as DHEA include ketoconazole and aminoglutethimide. Because the adrenal glands only make about 5% of the body's androgens, these medications are generally used only in combination with other methods that can block the 95% of androgens made by the testicles. These combined methods are called total androgen blockade (TAB). TAB can also be achieved using antiandrogens.
- GnRH action can be interrupted in one of two ways. GnRH antagonists suppress the production of LH directly, while GnRH agonists suppress LH through the process of downregulation after an initial stimulation effect. Abarelix is an example of a GnRH antagonist, while the GnRH agonists include leuprolide, goserelin, triptorelin, and buserelin. Initially, GnRH agonists increase the production of LH. However, because the constant supply of the medication does not match the body's natural production rhythm, production of both LH and GnRH decreases after a few weeks.[21]
- A very recent Trial I study (N=21) found that Abiraterone Acetate caused dramatic reduction in PSA levels and Tumor sizes in aggressive end-stage prostate cancer for 70% of patients. This is prostate cancer that resists all other treatments (e.g., castration, other hormones, etc.). Officially the impacts on life-span are not yet known because subjects have not been taking the drug very long. Larger Trial III Clinical Studies are in the works. If successful an approved treatment is hoped for around 2011.[22][23]
The most successful hormonal treatments are orchiectomy and GnRH agonists. Despite their higher cost, GnRH agonists are often chosen over orchiectomy for cosmetic and emotional reasons. Eventually, total androgen blockade may prove to be better than orchiectomy or GnRH agonists used alone.
Each treatment has disadvantages which limit its use in certain circumstances. Although orchiectomy is a low-risk surgery, the psychological impact of removing the testicles can be significant. The loss of testosterone also causes hot flashes, weight gain, loss of libido, enlargement of the breasts (gynecomastia), impotence and osteoporosis. GnRH agonists eventually cause the same side effects as orchiectomy but may cause worse symptoms at the beginning of treatment. When GnRH agonists are first used, testosterone surges can lead to increased bone pain from metastatic cancer, so antiandrogens or abarelix are often added to blunt these side effects. Estrogens are not commonly used because they increase the risk for cardiovascular disease and blood clots. The antiandrogens do not generally cause impotence and usually cause less loss of bone and muscle mass. Ketoconazole can cause liver damage with prolonged use, and aminoglutethimide can cause skin rashes.
Palliative care
Palliative care for advanced stage prostate cancer focuses on extending life and relieving the symptoms of metastatic disease. As noted above Abiraterone Acetate is showing some promise in treating advance stage prostate cancer. It causes a dramatic reduction in PSA levels and Tumor sizes in aggressive advanced-stage prostate cancer for 70% of patients. Chemotherapy may be offered to slow disease progression and postpone symptoms. The most commonly used regimen combines the chemotherapeutic drug docetaxel with a corticosteroid such as prednisone.[24] Bisphosphonates such as zoledronic acid have been shown to delay skeletal complications such as fractures or the need for radiation therapy in patients with hormone-refractory metastatic prostate cancer.[25]
Bone pain due to metastatic disease is treated with opioid pain relievers such as morphine and oxycodone. External beam radiation therapy directed at bone metastases may provide pain relief. Injections of certain radioisotopes, such as strontium-89, phosphorus-32, or samarium-153, also target bone metastases and may help relieve pain.
High Intensity Focused Ultrasound (HIFU)
HIFU for prostate cancer utilizes high intensity focused ultrasound (HIFU) to ablate/destroy the tissue of the prostate. During the HIFU procedure, sound waves are used to heat the prostate tissue thus destroying the cancerous cells. Essentially, ultrasonic waves are precisely focused on specific areas of the prostate to eliminate the prostate cancer with minimal risks of affecting other tissue or organs. Temperatures at the focal point of the sound waves can exceed 100 °C (212 °F).[26] In lay terms, the HIFU technology is similar to using a magnifying glass to burn a piece of paper by focusing sunlight at a small precise point on the sheet. The ability to focus the ultrasonic waves leads to a relatively low occurrence of both incontinence and impotence. (0.6% and 0-20%, respectively)[27] According to international studies, when compared to other procedures, HIFU has a high success rate with a reduced risk of side effects. Studies using the Sonablate 500 HIFU machine have shown that 94% of patients with a pretreatment PSA (Prostate Specific Antigen) of less than 10 ng/mL were cancer-free after three years.[27] However, many studies of HIFU were performed by manufacturers of HIFU devices, or members of manufacturers' advisory panels.[28]
HIFU was first used in the 1940s and 1950s in efforts to destroy tumors in the central nervous system. Since then, HIFU has been shown to be effective at destroying malignant tissue in the brain, prostate, spleen, liver, kidney, breast, and bone.[26] Today, the HIF procedure for prostate cancer is performed using a transrectal probe. This procedure has been performed for over ten years and is currently approved for use in Japan, Europe, Canada, and parts of Central and South America.
Although not yet approved for use in the Unites States, many patients have received the HIFU procedure at facilities in Canada, and Central and South America. Currently, therapy is available using the Sonablate 500 or the Ablatherm. The Sonablate 500 is designed by Focus Surgery of Indianapolis, Indiana and is used in international HIFU centers around the world.
Prognosis
Prostate cancer rates are higher and prognosis poorer in developed countries than the rest of the world. Many of the risk factors for prostate cancer are more prevalent in the developed world, including longer life expectancy and diets high in red meat and dairy products (although it must be noted, that people who consume larger amounts of meat and dairy, also tend to consume fewer portions of fruits and vegetables. It's not currently known whether or not both of this factors, or just one of them, contributes to the occurrence of prostate cancer).[29] Also, where there is more access to screening programs, there is a higher detection rate. Prostate cancer is the ninth most common cancer in the world, but is the number one non-skin cancer in United States men. Prostate cancer affected eighteen percent of American men and caused death in three percent in 2005.[30] In Japan, death from prostate cancer was one-fifth to one-half the rates in the United States and Europe in the 1990s.[31] In India in the 1990s, half of the people with prostate cancer confined to the prostate died within ten years.[32] African-American men have 50–60 times more prostate cancer and prostate cancer deaths than men in Shanghai, China.[33] In Nigeria, two percent of men develop prostate cancer and 64% of them are dead after two years.[34]
In patients who undergo treatment, the most important clinical prognostic indicators of disease outcome are stage, pre-therapy PSA level and Gleason score. In general, the higher the grade and the stage, the poorer the prognosis. Nomograms can be used to calculate the estimated risk of the individual patient. The predictions are based on the experience of large groups of patients suffering from cancers at various stages.[35]
Progression
In 1941, Charles Huggins reported that androgen ablation therapy causes regression of primary and metastatic androgen-dependent prostate cancer.[36] Androgen ablation therapy causes remission in 80-90% of patients undergoing therapy, resulting in a median progression-free survival of 12 to 33 months. After remission an androgen-independent phenotype typically emerges, where the median overall survival is 23–37 months from the time of initiation of androgen ablation therapy.[37] The actual mechanism contributes to the progression of prostate cancer is not clear and may vary between individual patient. A few possible mechanisms have been proposed.[38] Scientists have established a few prostate cancer cell lines to investigate the mechanism involved in the progression of prostate cancer. LNCaP, PC-3, and DU-145 are commonly used prostate cancer cell lines. The LNCaP cancer cell line was established from a human lymph node metastatic lesion of prostatic adenocarcinoma. PC-3 and DU-145 cells were established from human prostatic adenocarcinoma metastatic to bone and to brain, respectively. LNCaP cells express androgen receptor (AR), however, PC-3 and DU-145 cells express very little or no AR. AR, an androgen-activated transcription factor, belongs to the steroid nuclear receptor family. Development of the prostate is dependent on androgen signaling mediated through AR, and AR is also important during the development of prostate cancer. The proliferation of LNCaP cells is androgen-dependent but the proliferation of PC-3 and DU-145 cells is androgen-insensitive.Elevation of AR expression is often observed in advanced prostate tumors in patients.[39][40] Some androgen-independent LNCaP sublines have been developed from the ATCC androgen-dependent LNCaP cells after androgen deprivation for study of prostate cancer progression. These androgen-independent LNCaP cells have elevated AR expression and express prostate specific antigen upon androgen treatment. Androgens paradoxically inhibit the proliferation of these androgen-independent prostate cancer cells.[41][42][43] Androgen at a concentration of 10-fold higher than the physiological concentration has also been shown to cause growth suppression and reversion of androgen-independent prostate cancer xenografts or androgen-independent prostate tumors derived in vivo model to an androgen-stimulated phenotype in athymic mice.[44][45] These observation suggest the possibility to use androgen to treat the development of relapsed androgen-independent prostate tumors in patients. Oral infusion of green tea polyphenols, a potential alternative therapy for prostate cancer by natural compounds, has been shown to inhibit the development, progression, and metastasis as well in autochthonous transgenic adenocarcinoma of the mouse prostate (TRAMP) model, which spontaneously develops prostate cancer.[46]
Epidemiology
Rates of prostate cancer vary widely across the world. Although the rates vary widely between countries, it is least common in South and East Asia, more common in Europe, and most common in the United States.[47] According to the American Cancer Society, prostate cancer is least common among Asian men and most common among black men, with figures for white men in-between.[48][49] However, these high rates may be affected by increasing rates of detection.[50]
Prostate cancer develops most frequently in men over fifty. This cancer can occur only in men, as the prostate is exclusively of the male reproductive tract. It is the most common type of cancer in men in the United States, where it is responsible for more male deaths than any other cancer, except lung cancer. In the United Kingdom it is also the second most common cause of cancer death after lung cancer, where around 35,000 cases are diagnosed every year and of which around 10,000 die of it. However, many men who develop prostate cancer never have symptoms, undergo no therapy, and eventually die of other causes. That is because malignant neoplasms of the prostate are, in most cases, slow-growing, and because most of those affected are over 60. Hence they often die of causes unrelated to the prostate cancer, such as heart/circulatory disease, pneumonia, other unconnected cancers or old age. Many factors, including genetics and diet, have been implicated in the development of prostate cancer. The Prostate Cancer Prevention Trial found that finasteride reduces the incidence of prostate cancer rate by 30%. There had been a controversy about this also increasing the risk of more aggressive cancers, but more recent research showed this was not the case.[51][52]
History

Although the prostate was first described by Venetian anatomist Niccolò Massa in 1536, and illustrated by Flemish anatomist Andreas Vesalius in 1538, prostate cancer was not identified until 1853.[53] Prostate cancer was initially considered a rare disease, probably because of shorter life expectancies and poorer detection methods in the 19th century. The first treatments of prostate cancer were surgeries to relieve urinary obstruction.[54] Removal of the entire gland (radical perineal prostatectomy) was first performed in 1904 by Hugh H. Young at Johns Hopkins Hospital.[55] Surgical removal of the testes (orchiectomy) to treat prostate cancer was first performed in the 1890s, but with limited success. Transurethral resection of the prostate (TURP) replaced radical prostatectomy for symptomatic relief of obstruction in the middle of the 20th century because it could better preserve penile erectile function. Radical retropubic prostatectomy was developed in 1983 by Patrick Walsh.[56] This surgical approach allowed for removal of the prostate and lymph nodes with maintenance of penile function.
In 1941 Charles B. Huggins published studies in which he used estrogen to oppose testosterone production in men with metastatic prostate cancer. This discovery of "chemical castration" won Huggins the 1966 Nobel Prize in Physiology or Medicine.[57] The role of the hormone GnRH in reproduction was determined by Andrzej W. Schally and Roger Guillemin, who both won the 1977 Nobel Prize in Physiology or Medicine for this work.
Receptor agonists, such as leuprolide and goserelin, were subsequently developed and used to treat prostate cancer.[58][59]
Radiation therapy for prostate cancer was first developed in the early 20th century and initially consisted of intraprostatic radium implants. External beam radiation became more popular as stronger radiation sources became available in the middle of the 20th century. Brachytherapy with implanted seeds was first described in 1983.[60] Systemic chemotherapy for prostate cancer was first studied in the 1970s. The initial regimen of cyclophosphamide and 5-fluorouracil was quickly joined by multiple regimens using a host of other systemic chemotherapy drugs.[61]
Histopathological Findings in Prostatic Adenocarcinoma
Prostate: Adenocarcinoma
<youtube v=1SZPLS1dxTo/>
Prostate: Adenocarcinoma (Gleason grading system)
Prostate: Adenocarcinoma (Gleason grade 1)
<youtube v=F7V0Zl7a2FY/>
Prostate : Adenocarcinoma (Gleason grade 2)
<youtube v=YSOLiSklIXw/>
Prostate : Adenocarcinoma (Gleason grade 3)
<youtube v=TG8vR_pE7yA/>
Prostate: Adenocarcinoma (Gleason grade 4)
<youtube v=R2Cl4HScdGc/>
Prostate: Adenocarcinoma (Gleason grade 5)
<youtube v=F7V0Zl7a2FY/>
See also
References
- ↑ 1.0 1.1 Smith JA, Chan RC, Chang SS; et al. (2007). "A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy". J. Urol. 178 (6): 2385–9, discussion 2389–90. doi:10.1016/j.juro.2007.08.008. PMID 17936849.
- ↑ Mouraviev V, Evans B, Polascik TJ (2006). "Salvage prostate cryoablation after primary interstitial brachytherapy failure: a feasible approach". Prostate Cancer Prostatic Dis. 9 (1): 99–101. doi:10.1038/sj.pcan.4500853. PMID 16314889.
- ↑ Wu, H (2004). "Watchful waiting and factors predictive of secondary treatment of localized prostate cancer". J Urol. 171 (3): 1111–6. doi:10.1097/01.ju.0000113300.74132.8b. PMID 14767282. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) - ↑ Ornish, D (2005). "Intensive lifestyle changes may affect the progression of prostate cancer". J Urol. 174 (3): 1065–70. PMID 16094059. Unknown parameter
|coauthors=ignored (help) - ↑ Frattaroli, J (2008). "Clinical events in Prostate CAncer Lifestyle Trial: Results from two years of follow-up". Urology. epub ahead of print. PMID 18602144. Unknown parameter
|coauthors=ignored (help); Unknown parameter|month=ignored (help) - ↑ Pantuck, AJ (2006). "Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer". Clin Cancer Res. 12 (13): 4018–26. PMID 16818701. Unknown parameter
|coauthors=ignored (help) - ↑ Kumar, NB (2004). "The specific role of isoflavones in reducing prostate cancer risk". Prostate. 59 (2): 141–7. PMID 15042614. Unknown parameter
|coauthors=ignored (help) - ↑ Yarnell, E (1999). "A naturopathic approach to prostate cancer. Part 2: Guidelines for treatment and prevention". Altern Complemen Ther. 5 (6): 360–8.
- ↑ Bill-Axelson A, Holmberg L, Ruutu M; et al. (2005). "Radical prostatectomy versus watchful waiting in early prostate cancer". N. Engl. J. Med. 352 (19): 1977–84. doi:10.1056/NEJMoa043739. PMID 15888698.
- ↑ Gerber, GS (1996). "Results of radical prostatectomy in men with clinically localized prostate cancer". JAMA. 276 (8): 615–9. doi:10.1001/jama.276.8.615. PMID 8773633. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) - ↑ Nag, S (1999). "American Brachytherapy Society Recommendations for Transperineal Permanent Brachytherapy of Prostate Cancer". Int. J. Rad. Onc. Biol. Phys. 44 (4): 789–799. ?. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) Review. - ↑ Perez, CA (1993). "Localized carcinoma of the prostate (stages T1B, T1C, T2, and T3). Review of management with external beam radiation therapy". Cancer. 72 (11): 3156–73. doi:10.1002/1097-0142(19931201)72:11<3156::AID-CNCR2820721106>3.0.CO;2-G. PMID 7694785. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) Review. - ↑ D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW (2004). "6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial". JAMA. 292 (7): 821–7. doi:10.1001/jama.292.7.821. PMID 15315996.
- ↑ American Cancer Society: Radiation Treatment
- ↑ Lawton, CA (1991). "Long-term treatment sequelae following external beam irradiation for adenocarcinoma of the prostate: analysis of RTOG studies 7506 and 7706". Int J Radiat Oncol Biol Phys. 21 (4): 935–9. PMID 1917622. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) - ↑ Brenner, DJ (2000). "Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery". Cancer. 88 (2): 398–406. doi:10.1002/(SICI)1097-0142(20000115)88:2<398::AID-CNCR22>3.0.CO;2-V. PMID 10640974. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) - ↑ PreventProstateCancer.info: A Brief Overview of Prostate Cancer [1]
- ↑ "Cryosurgical system for destroying tumors by freezing". Retrieved 1994-08-02. Check date values in:
|accessdate=(help) - ↑ Bahn, DK (2002). "Targeted cryoablation of the prostate: 7-year outcomes in the primary treatment of prostate cancer". Urology. 60 (2 Suppl 1): 3–11. doi:10.1016/S0090-4295(02)01678-3. PMID 12206842. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) - ↑ Robson, M (1996). "How is androgen-dependent metastatic prostate cancer best treated?". Hematol Oncol Clin North Am. 10 (3): 727–47. doi:10.1016/S0889-8588(05)70364-6. PMID 8773508. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) Review. - ↑ Loblaw, DA (2004). "American Society of Clinical Oncology recommendations for the initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer". J Clin Oncol. 22 (14): 2927–41. doi:10.1200/JCO.2004.04.579. PMID 15184404. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) Erratum in: J Clin Oncol. 2004 November 1;22(21):4435. - ↑ de Bono, Johann (2004). "Phase I Clinical Trial of a Selective Inhibitor of CYP17, Abiraterone Acetate, Confirms That Castration-Resistant Prostate Cancer Commonly Remains Hormone Driven". J Clin Oncol: online. doi:10.1200/JCO.2007.15.9749. PMID 15184404. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) Erratum in: J Clin Oncol. Early Release, published ahead of print July 21, 2008 - ↑ Richard Warry (July 22, 2008). "Drug for deadly prostate cancer". BBC. Retrieved 2008-07-23.
- ↑ Tannock, IF (2004). "Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer". N Engl J Med. 351 (15): 1502–12. doi:10.1056/NEJMoa040720. PMID 1547021. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) - ↑ Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Chen B (2002). "A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma". J Natl Cancer Inst. 94 (19): 1458–68. PMID 12359855.
- ↑ 26.0 26.1 Thomas A. Gardner and Michael A Koch, Indiana University Medical Center, Indianapolis. Prostate Cancer Therapy with High-Intensity Focused Ultrasound-Comprehensive Review. Clinical Genitourinary Cancer Vol 4. No.3, 2005.
- ↑ 27.0 27.1 Toyoaki Uchida, et al. Five years experience of transrectal high-intensity focused ultrasound using the Sonablate device in the treatment of localized prostate cancer. Dept of Urology University of Tokai Hachioji Hospital. International Journal of Urology
- ↑ Tom Pickles, Larry Goldenberg, Gary Steinhoff. High Intensity Focused Ultrasound for Prostate Cancer. British Columbia Cancer Agency http://www.bccancer.bc.ca/NR/rdonlyres/08EA1C8E-4345-4C7E-A83A-1F84853A1C27/8101/HIFUreport2005Feb10revised1.pdf
- ↑ ACS :: What Are The Risk Factors for Prostate Cancer?
- ↑ Jemal, A (2005). "Cancer statistics, 2005". CA Cancer J Clin. 55 (1): 10–30. PMID 15661684. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) Erratum in: CA Cancer J Clin. 2005 Jul-Aug;55(4):259. - ↑ Wakai, K (2005). "Descriptive epidemiology of prostate cancer in Japan and Western countries". Nippon Rinsho. 63 (2): 207–12. PMID 15714967. Unknown parameter
|month=ignored (help) Review. Template:Ja icon - ↑ Yeole, BB (2001). "Population based survival from prostate cancer in Mumbai (Bombay), India". Indian J Cancer. 38 (2–4): 126–32. PMID 1259345. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) - ↑ Hsing, AW (2000). "International trends and patterns of prostate cancer incidence and mortality". Int J Cancer. 85 (1): 60–7. doi:10.1002/(SICI)1097-0215(20000101)85:1<60::AID-IJC11>3.0.CO;2-B. PMID 10585584. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) - ↑ Osegbe, DN (1997). "Prostate cancer in Nigerians: facts and nonfacts". J Urol. 157 (4): 1340–3. doi:10.1016/S0022-5347(01)64966-8. PMID 9120935. Unknown parameter
|month=ignored (help) - ↑ Di Blasio CJ, Rhee AC, Cho D, Scardino PT, Kattan MW (2003). "Predicting clinical end points: treatment nomograms in prostate cancer". Semin Oncol. 30 (5): 567–86. doi:10.1016/S0093-7754(03)00351-8. PMID 14571407.
- ↑ Huggins C, Steven RE and Hodges CV, Studies on prostatic cancer. Arch. Sug. 43:209–223, 1941.
- ↑ Hellerstedt BA and Pienta KJ, The current state of hormonal therapy for prostate cancer, CA Cancer J. Clin. 52: 154–179, 2002.PMID 12018929
- ↑ Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001 Oct;1(1):34–45. PMID 11900250
- ↑ Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001 May 1;61(9):3550–5. PMID 11325816
- ↑ Ford OH 3rd, Gregory CW, Kim D, Smitherman AB, Mohler JL. Androgen receptor gene amplification and protein expression in recurrent prostate cancer. J Urol. 2003 Nov;170(5):1817–21.PMID 14532783
- ↑ Kokontis J, Takakura K, Hay N, Liao S. Increased androgen receptor activity and altered c-myc expression in prostate cancer cells after long-term androgen deprivation. Cancer Res. 1994 March 15;54(6):1566–73. PMID 7511045
- ↑ Umekita Y, Hiipakka RA, Kokontis JM, Liao S. Human prostate tumor growth in athymic mice: inhibition by androgens and stimulation by finasteride. Proc Natl Acad Sci U S A. 1996 October 15;93(21):11802-7. PMID 8876218
- ↑ Kokontis JM, Hsu S, Chuu CP, Dang M, Fukuchi J, Hiipakka RA, Liao S. Role of androgen receptor in the progression of human prostate tumor cells to androgen independence and insensitivity. Prostate. 2005 December 1;65(4):287-98. PMID 16015608
- ↑ Chuu CP, Hiipakka RA, Fukuchi J, Kokontis JM, Liao S. Androgen causes growth suppression and reversion of androgen-independent prostate cancer xenografts to an androgen-stimulated phenotype in athymic mice. Cancer Res. 2005 March 15;65(6):2082–4. PMID 15781616
- ↑ Chuu CP, Hiipakka RA, Kokontis JM, Fukuchi J, Chen RY, Liao S. Inhibition of tumor growth and progression of LNCaP prostate cancer cells in athymic mice by androgen and liver X receptor agonist. Cancer Res. 2006 July 1;66(13):6482–6. PMID 16818617
- ↑ Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci U S A. 2001 August 28;98(18):10350-5. PMID 11504910
- ↑ "IARC Worldwide Cancer Incidence Statistics—Prostate". JNCI Cancer Spectrum. Oxford University Press. December 19, 2001. Retrieved on 2007-04-05 through the Internet Archive
- ↑ Overview: Prostate Cancer—What Causes Prostate Cancer? American Cancer Society (2006-05-02). Retrieved on 2007-04-05
- ↑ Prostate Cancer FAQs. State University of New York School of Medicine Department of Urology (2006-08-31). Retrieved on 2007-04-05
- ↑ Potosky A, Miller B, Albertsen P, Kramer B (1995). "The role of increasing detection in the rising incidence of prostate cancer". JAMA. 273 (7): 548&ndash, 52. doi:10.1001/jama.273.7.548. PMID 7530782.
- ↑ Gine Kolata (June 15, 2008). "New Take on a Prostate Drug, and a New Debate". NY Times. Retrieved 2008-06-15.
- ↑ Potosky A, Miller B, Albertsen P, Kramer B (2008). "Finasteride Does Not Increase the Risk of High-Grade Prostate Cancer: A Bias-Adjusted Modeling Approach". Cancer Prevention Research. Published Online First on May 18, 2008 as 10.1158/1940-6207.CAPR-08-0092: 174. doi:10.1158/1940-6207.CAPR-08-0092.
- ↑ Adams, J. The case of scirrhous of the prostate gland with corresponding affliction of the lymphatic glands in the lumbar region and in the pelvis. Lancet 1, 393 (1853).
- ↑ Lytton, B. Prostate cancer: a brief history and the discovery of hormonal ablation treatment. J. Urol. 165, 1859–1862
- ↑ Young, H. H. Four cases of radical prostatectomy. Johns Hopkins Bull. 16, 315 (1905).
- ↑ Walsh, P. C., Lepor, H. & Eggleston, J. C. Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate 4, 473-485 (1983). PMID 6889192
- ↑ Huggins, C. B. & Hodges, C. V. Studies on prostate cancer: 1. The effects of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1, 203 (1941).
- ↑ Schally, A. V., Kastin, A. J. & Arimura, A. Hypothalamic FSH and LH-regulating hormone. Structure, physiology and clinical studies. Fertil. Steril. 22, 703–721 (1971).
- ↑ Tolis G, Ackman D, Stellos A, Mehta A, Labrie F, Fazekas AT, Comaru-Schally AM, Schally AV. Tumor growth inhibition in patients with prostatic carcinoma treated with luteinizing hormone-releasing hormone agonists. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1658–62 PMID 6461861
- ↑ Denmeade SR, Isaacs JT. A History of Prostate Cancer Treatment. Nature Reviews Cancer 2, 389–396 (2002). PMID 12044015
- ↑ Scott, W. W. et al. Chemotherapy of advanced prostatic carcinoma with cyclophosphamide or 5-fluorouracil: results of first national randomized study. J. Urol. 114, 909–911 (1975). PMID 1104900
External links
Template:Urogenital neoplasia Template:SIB Template:Link FA Template:Link FA af:Prostaatkanker bn:প্রোস্টেট ক্যান্সার bg:Рак на простатата ca:Càncer de pròstata da:Prostatakræft de:Prostatakrebs dv:ޕްރޮސްޓޭޓް ކެންސަރު el:Καρκίνος του προστάτη fa:سرطان پروستات hr:Rak prostate id:Kanker prostat it:Carcinoma della prostata he:סרטן הערמונית la:Cancer prostatae lv:Prostatas vēzis nl:Prostaatkanker no:Prostatakreft simple:Prostate cancer fi:Eturauhassyöpä sv:Prostatacancer tl:Kanser sa prostata