Lidocaine (injection): Difference between revisions
No edit summary |
m (Protected "Lidocaine (injection)": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (18 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{DrugProjectFormSinglePage | ||
|authorTag={{Alonso}} | |||
|genericName=Lidocaine | |||
|aOrAn=a | |||
|drugClass=[[antiarrhythmic]], local [[anesthetic]] | |||
|indicationType=treatment | |||
|indication=[[ventricular arrhythmias]] such as those occurring in relation to acute [[myocardial infarction]], or during cardiac manipulation, such as [[cardiac surgery]] | |||
|adverseReactions=[[bradyarrhythmia]], [[hypotension]], [[backache]] [[dizziness]], [[headache]], [[lightheadedness]], [[numbness]], [[paresthesia]], [[shivering]], [[somnolence]], blurred vision, burning sensation in eye, [[conjunctival hyperemia]], corneal epithelial defect, [[diplopia]], [[apprehension]], [[confusion]], [[euphoria]], feeling nervous. | |||
|blackBoxWarningTitle=Warning Title | |||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |||
|fdaLIADAdult======Single Direct Intravenous Injection (bolus)===== | |||
* Only the 50 and 100 mg dosage sizes should be used for direct intravenous injection. The usual dose is 50 to 100 mg of lidocaine hydrochloride (0.70 to 1.4 mg/kg; 0.32 to 0.63 mg/lb) administered intravenously under [[ECG monitoring]]. This dose may be administered at the rate of approximately 25 to 50 mg/min (0.35 to 0.70 mg/kg/min; 0.16 to 0.32 mg/lb/min). | |||
* Sufficient time should be allowed to enable a slow circulation to carry the drug to the site of action. If the initial injection of 50 to 100 mg does not produce a desired response, a second dose may be injected after 5 minutes. No more than 200 to 300 mg of lidocaine hydocloride should be administered during a one hour period. | |||
=====Continuous Intravenous Infusion===== | |||
* Following bolus administration, intravenous infusions of lidocaine hydrochloride may be initiated at the rate of 1 to 4 mg/min of lidocaine hydrochloride (0.014 to 0.057 mg/kg/min; 0.006 to 0.026 mg/lb/min). The rate of intravenous infusions should be reassessed as soon as the patient’s basic [[cardiac rhythm]] appears to be stable or at the earliest signs of toxicity. It should rarely be necessary to continue intravenous infusions of lidocaine for prolonged periods. | |||
* Solutions for intravenous infusion may be prepared by the addition of one gram (or two grams) of lidocaine hydrochloride to one liter of 5% dextrose in water using aseptic technique. Approximately a 0.1% (or 0.2%) solution will result from this procedure; that is, each milliliter will contain approximately 1 (or 2) mg of lidocaine hydrochloride. In those cases in which fluid restriction is medically appropriate, a more concentrated solution may be prepared. | |||
* Lidocaine hydrochloride injection has been found to be chemically stable for 24 hours after dilution in 5% dextrose in water. However, as with all intravenous admixtures, dilution of the solution should be made just prior to its administration. | |||
* It is very important that after adding lidocaine hydrochloride, or any other medication, to an I.V. container, the contents be thoroughly mixed before beginning the infusion. | |||
* When administering by continuous I.V. infusion, it is advisable to use a precision volume control I.V. set. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|offLabelAdultNoGuideSupport======Aortocoronary Bypass Grafting===== | |||
* Dosing Information | |||
:* 1 mg/min IV infusion<ref name="pmid6981016">{{cite journal| author=Sunamori M, Okamura T, Amano J, Suma H, Suzuki A| title=Myocardial protection by lidocaine hydrochloride in aorto-coronary bypass surgery. | journal=Jpn J Surg | year= 1982 | volume= 12 | issue= 2 | pages= 93-7 | pmid=6981016 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=6981016 }} </ref> | |||
:* 100 mg bolus 2 minutes before releasing the aortic clamp administered through a bypass pump.<ref name="pmid11052433">{{cite journal| author=Baraka A, Kawkabani N, Dabbous A, Nawfal M| title=Lidocaine for prevention of reperfusion ventricular fibrillation after release of aortic cross-clamping. | journal=J Cardiothorac Vasc Anesth | year= 2000 | volume= 14 | issue= 5 | pages= 531-3 | pmid=11052433 | doi=10.1053/jcan.2000.9484 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11052433 }} </ref> | |||
=====Regional Pain Syndrome===== | |||
* Dosing Information | |||
:* Lidocaine continuous infusion administered as a initial dose of 200 mg through the first hour, followed by 100 to 190 mg/h according to tolerance. Infusion should be continue until maximum pain control is reached.<ref name="pmid10206569">{{cite journal| author=Linchitz RM, Raheb JC| title=Subcutaneous infusion of lidocaine provides effective pain relief for CRPS patients. | journal=Clin J Pain | year= 1999 | volume= 15 | issue= 1 | pages= 67-72 | pmid=10206569 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10206569 }} </ref> | |||
=====Infusion Pain===== | |||
* Dosing Information | |||
:* Addition to methohexital and propofol, concentrations: lidocaine 0.1% to 1%.<ref name="pmid10470630">{{cite journal| author=Ho CM, Tsou MY, Sun MS, Chu CC, Lee TY| title=The optimal effective concentration of lidocaine to reduce pain on injection of propofol. | journal=J Clin Anesth | year= 1999 | volume= 11 | issue= 4 | pages= 296-300 | pmid=10470630 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10470630 }} </ref><ref name="pmid9175962">{{cite journal| author=Eriksson M, Englesson S, Niklasson F, Hartvig P| title=Effect of lignocaine and pH on propofol-induced pain. | journal=Br J Anaesth | year= 1997 | volume= 78 | issue= 5 | pages= 502-6 | pmid=9175962 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9175962 }} </ref> | |||
=====Cough===== | |||
* Dosing Information | |||
:* 1 to 2 mg/kg.<ref name="pmid6837243">{{cite journal| author=Gefke K, Andersen LW, Friesel E| title=Lidocaine given intravenously as a suppressant of cough and laryngospasm in connection with extubation after tonsillectomy. | journal=Acta Anaesthesiol Scand | year= 1983 | volume= 27 | issue= 2 | pages= 111-2 | pmid=6837243 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=6837243 }} </ref><ref name="pmid3347458">{{cite journal| author=Stewart RH, Kimbrough RL, Engstrom PF, Cameron B| title=Lidocaine: an anti-tussive for ophthalmic surgery. | journal=Ophthalmic Surg | year= 1988 | volume= 19 | issue= 2 | pages= 130-1 | pmid=3347458 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3347458 }} </ref> | |||
=====Elective Abortion===== | |||
* Dosing Information | |||
:* 7 to 30 mL of 1% lidocaine administered through the umbilical vein, associated to 5 mcg of sufentanil, both administered 48 hours following mifepristone treatment (600 mg).<ref name="pmid12628271">{{cite journal| author=Senat MV, Fischer C, Bernard JP, Ville Y| title=The use of lidocaine for fetocide in late termination of pregnancy. | journal=BJOG | year= 2003 | volume= 110 | issue= 3 | pages= 296-300 | pmid=12628271 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12628271 }} </ref> | |||
=====Fibromyalgia===== | |||
* Dosing Information | |||
:* Administer an initial dose of 5 mg/kg minus 100 mg, followed by 50 mg/day increases up to 5 mg/kg plus 150 mg. Do not excede 550 mg infused. Infusion should be administered over 6 hours, diluted in 500 mL of Hartman's solution.<ref name="pmid12217079">{{cite journal| author=Raphael JH, Southall JL, Treharne GJ, Kitas GD| title=Efficacy and adverse effects of intravenous lignocaine therapy in fibromyalgia syndrome. | journal=BMC Musculoskelet Disord | year= 2002 | volume= 3 | issue= | pages= 21 | pmid=12217079 | doi= | pmc=PMC126218 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12217079 }} </ref> | |||
=====Indigestion===== | |||
* Dosing Information | |||
:* 30 mL of antacid associated with 15 mL of 2% viscous lidocaine, both administered simultaneously PO.<ref name="pmid2202240">{{cite journal| author=Welling LR, Watson WA| title=The emergency department treatment of dyspepsia with antacids and oral lidocaine. | journal=Ann Emerg Med | year= 1990 | volume= 19 | issue= 7 | pages= 785-8 | pmid=2202240 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2202240 }} </ref> | |||
=====Peritubular Block===== | |||
* Dosing Information | |||
:* 5 mL of lidocaine (2%) plus 5 mL bupivacaine 0.75% with 150 IU of hyaluronidase.<ref name="pmid9038442">{{cite journal| author=Gao F, Budd AJ| title=Venous levels of lignocaine and bupivacaine after peribulbar block. | journal=Anaesthesia | year= 1996 | volume= 51 | issue= 12 | pages= 1109-12 | pmid=9038442 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9038442 }} </ref> | |||
=====Tumescent Anesthesia-Liposuction Procedure===== | |||
* Dosing Information | |||
:* Administer a solution of: lidocaine 500 to 1000 mg + epinephrine 0.5 mg + sodium bicarbonate 10 mEq + triamcinolone 10 mg + 1 liter saline solution 0.09%. Solution should be administered directly into the subcutaneous tissue undergoing the procedure at a rate of 150 mL/hour. Administer over an average time of 90 to 120 minutes.<ref name="pmid9063507">{{cite journal| author=Ostad A, Kageyama N, Moy RL| title=Tumescent anesthesia with a lidocaine dose of 55 mg/kg is safe for liposuction. | journal=Dermatol Surg | year= 1996 | volume= 22 | issue= 11 | pages= 921-7 | pmid=9063507 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9063507 }} </ref> | |||
=====Postoperative Pain===== | |||
* Dosing Information | |||
:* Administer 1.5 mg/kg 30 minutes before surgery, followed by a 1.5 mg/kg/hour continuous infusion through the first hour following surgery.<ref name="pmid15041597">{{cite journal| author=Koppert W, Weigand M, Neumann F, Sittl R, Schuettler J, Schmelz M et al.| title=Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery. | journal=Anesth Analg | year= 2004 | volume= 98 | issue= 4 | pages= 1050-5, table of contents | pmid=15041597 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15041597 }} </ref> | |||
=====Seizure===== | |||
* Dosing Information | |||
:* Administer 1.5 to 2 mg/kg IV infucion over 2 minutes. If seizure recurrence is observed dosage can be repeated.<ref name="pmid3409844">{{cite journal| author=Pascual J, Sedano MJ, Polo JM, Berciano J| title=Intravenous lidocaine for status epilepticus. | journal=Epilepsia | year= 1988 | volume= 29 | issue= 5 | pages= 584-9 | pmid=3409844 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3409844 }} </ref> | |||
=====Tinnitus===== | |||
* Dosing Information | |||
:* 1.5 mg/kg IV.<ref name="pmid7049137">{{cite journal| author=Israel JM, Connelly JS, McTigue ST, Brummett RE, Brown J| title=Lidocaine in the treatment of tinnitus aurium. A double-blind study. | journal=Arch Otolaryngol | year= 1982 | volume= 108 | issue= 8 | pages= 471-3 | pmid=7049137 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7049137 }} </ref> | |||
|fdaLIADPed=Controlled clinical studies in the pediatric population to establish dosing schedules have not been conducted. The American Heart Association’s Standards and Guidelines recommends a bolus dose of 1 mg/kg, and an infusion rate of between 20 to 50 mcg/kg/min for prolonged therapy. When drug clearance is reduced, as in patients with shock, [[congestive heart failure]] or [[cardiac arrest]], the infusion rate should not exceed 20 mcg/kg/min. | |||
'''Note Regarding Prolonged Infusion:''' There are data that indicate the half-life may be 3 hours or longer following infusions of greater than 24 hours in duration. | |||
'''Note:''' Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever the solution and container permit. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Lidocaine in pediatric patients. | |||
|offLabelPedNoGuideSupport======Seizure===== | |||
* Dosing Information | |||
:* Initial dose of 4 to 6 mg/kg/hr, followed by infusions of 4 to 8 mg/kg/hr administered over 11 to 60 hours until successful response was achieved.<ref name="pmid23738612">{{cite journal| author=Lundqvist M, Ågren J, Hellström-Westas L, Flink R, Wickström R| title=Efficacy and safety of lidocaine for treatment of neonatal seizures. | journal=Acta Paediatr | year= 2013 | volume= 102 | issue= 9 | pages= 863-7 | pmid=23738612 | doi=10.1111/apa.12311 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23738612 }} </ref> | |||
|contraindications=* [[Hypersensitivity]] to local anesthetics of the amide type. | |||
* [[Stokes-Adams syndrome]]. | |||
* [[Wolff-Parkinson-White syndrome]]. | |||
* Patients with severe degrees of sinoatrial, atrioventricular, or intraventricular block in the absence of an artificial pacemaker. | |||
|warnings=* Systemic toxicity may result in manifestations of [[central nervous system depression]] ([[sedation]]) or [[irritability]] (twitching), which may progress to frank [[convulsions]] accompanied by [[respiratory depression]] and/or [[Respiratory depression|arrest]]. Early recognition of premonitory signs, assurance of adequate oxygenation and, where necessary, establishment of artificial airway with [[ventilatory support]] are essential to management of this problem. Should [[convulsions]] persist despite [[ventilatory therapy]] with oxygen, small increments of [[anticonvulsant drugs]] may be used intravenously. Examples of such agents include [[benzodiazepines]] (e.g., [[diazepam]]), ultra short-acting [[barbiturates]] (e.g., [[thiopental]] or [[thiamylal]]), or a short-acting [[barbiturate]] (e.g., [[pentobarbital]] or [[secobarbital]]). If the patient is under anesthesia, a short-acting muscle relaxant (e.g., [[succinylcholine]]) may be used. Longer acting drugs should be used only when recurrent convulsions are evidenced. | |||
* Should circulatory depression occur, [[vasopressors]] may be used. | |||
* Constant [[electrocardiographic]] monitoring is essential to the proper administration of lidocaine hydrochloride. Signs of excessive depression of cardiac electrical activity such as sinus node dysfunction, prolongation of the P-R interval and QRS complex or the appearance or aggravation of [[arrhythmias]], should be followed by flow adjustment and, if necessary, prompt cessation of the intravenous infusion of this agent. Occasionally, acceleration of ventricular rate may occur when lidocaine hydrochloride is administered to patients with [[atrial flutter]] or [[atrial fibrillation]]. | |||
====Precautions==== | |||
=====General===== | |||
* Caution should be employed in the use of lidocaine hydrochloride in patients with severe [[liver disease]] or [[kidney disease]] because accumulation of the drug or metabolites may occur. | |||
* Lidocaine hydrochloride should be used with caution in the treatment of patients with [[hypovolemia]], severe [[congestive heart failure]], shock, and all forms of [[heart block]]. In patients with sinus [[bradycardia]] or incomplete [[heart block]], the administration of lidocaine hydrochloride intravenously for the elimination of ventricular ectopic beats, without prior acceleration in [[heart rate]] (e.g., by [[atropine]], [[isoproterenol]] or electric pacing), may promote more frequent and serious [[ventricular arrhythmias]] or complete [[heart block]]. | |||
* Dosage should be reduced for children and for debilitated and/or elderly patients, commensurate with their age and physical status. | |||
* The safety of amide local anesthetic agents in patients with genetic predisposition to malignant [[hyperthermia]] has not been fully assessed; therefore, lidocaine should be used with caution in such patients. | |||
* In hospital environments where drugs known to be triggering agents for malignant [[hyperthermia]] (fulminant hypermetabolism) are administered, it is suggested that a standard protocol for management should be available. | |||
* It is not known whether lidocaine may trigger this reaction; however, large doses resulting in significant plasma concentrations, as may be achieved by intravenous infusion, pose potential risk to these individuals. Recognition of early unexplained signs of [[tachycardia]], [[tachypnea]], labile [[blood pressure]] and [[metabolic acidosis]] may precede temperature elevation. Successful outcome is dependent on early diagnosis, prompt discontinuance of the triggering agent and institution of treatment including oxygen therapy, supportive measures and dantrolene. | |||
|clinicalTrials=Adverse experiences following the administration of lidocaine are similar in nature to those observed with other amide local anesthetic agents. Adverse experiences may result from high plasma levels caused by excessive dosage or may result from a [[hypersensitivity]], idiosyncrasy or diminished tolerance on the part of the patient. Serious adverse experiences are generally systemic in nature. The following types are those most commonly reported. The adverse experiences under [[Central Nervous System]] and [[Cardiovascular System]] are listed, in general, in a progression from mild to severe. | |||
=====Central Nervous System===== | |||
CNS reactions are excitatory and/or depressant, and may be characterized by [[lightheadedness]], [[nervousness]], apprehension, [[euphoria]], [[confusion]], [[dizziness]], [[drowsiness]], [[tinnitus]], blurred or double vision, [[vomiting]], sensations of heat, cold or numbness, twitching, [[tremors]], [[convulsions]], [[unconsciousness]], [[respiratory depression]] and [[respiratory arrest]]. The excitatory reactions may be very brief or may not occur at all, in which case, the first manifestation of toxicity may be [[drowsiness]], merging into [[unconsciousness]] and [[respiratory arrest]]. | |||
=====Cardiovascular System===== | |||
Cardiovascular reactions are usually depressant in nature and are characterized by [[bradycardia]], [[hypotension]], and cardiovascular collapse, which may lead to [[cardiac arrest]]. | |||
=====Allergic Reactions===== | |||
[[Allergic reactions]] as a result of sensitivity to lidocaine are extremely rare and, if they occur, should be managed by conventional means. | |||
=====Drug Abuse or Dependance===== | |||
Although specific studies have not been conducted, lidocaine hydrochloride has been used clinically without evidence of abuse of this drug or of physiological or physical dependence as a result of its use. | |||
|drugInteractions=* Lidocaine hydrochloride should be used with caution in patients with [[digitalis]] toxicity accompanied by [[atrioventricular block]]. | |||
* Concomitant use of [[beta blockers]] may reduce hepatic blood flow and thereby reduce lidocaine clearance. | |||
* Lidocaine and [[tocainide]] are pharmacologically similar. | |||
* The concomitant use of these two agents may cause an increased incidence of adverse reactions, including [[central nervous system]] adverse reactions such as [[seizure]]. | |||
|FDAPregCat=B | |||
|useInPregnancyFDA=Reproduction studies have been performed in rats at doses up to 6.6 times the maximum human doses and have revealed no significant findings. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predicted of human response, this drug should be used during pregnancy only if clearly needed. | |||
|useInLaborDelivery=The effects of lidocaine hydrochloride on the mother and the fetus, when used in the management of cardiac arrhythmias during labor and delivery, are not known. Lidocaine readily crosses the placental barrier. | |||
|useInNursing=It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when lidocaine is administered to a nursing woman. | |||
|useInPed=Safety and effectiveness in children have not been established by controlled clinical studies. | |||
|administration=* Intravenous | |||
|monitoring=Lidocaine hydrochloride is administered intravenously under [[ECG monitoring]]. | |||
|IVCompat=There is limited information regarding the IV compatibility provided by the label. | |||
|overdose=* Overdosage of lidocaine hydrochloride usually results in signs of [[central nervous system]] or [[cardiovascular system]] toxicity. | |||
* Should convulsions or signs of [[respiratory depression]] and [[respiratory arrest]] develop, the patency of the airway and adequacy of ventilation must be assured immediately. Should [[convulsions]] persist despite ventilatory therapy with oxygen, small increments of [[anticonvulsive agents]] may be given intravenously. Examples of such agents include a [[benzodiazepine]] (e.g., [[diazepam]]), an ultrashort-acting [[barbiturate]] (e.g., [[thiopental]] or [[thiamylal]]), or a short-acting barbiturate (e.g., [[pentobarbital]] or [[secobarbital]]). If the patient is under general anesthesia, a short-acting muscle relaxant (e.g., [[succinylcholine]]) may be administered. | |||
* Should circulatory depression occur, [[vasopressors]] may be used. Should cardiac arrest occur, standard [[CPR]] procedures should be instituted. | |||
* [[Dialysis]] is of negligible value in the treatment of acute overdosage from lidocaine hydrochloride. | |||
|drugBox={{Drugbox2 | |||
| verifiedrevid = 464370713 | | verifiedrevid = 464370713 | ||
| IUPAC_name = 2-(diethylamino)-<br>''N''-(2,6-dimethylphenyl)acetamide | | IUPAC_name = 2-(diethylamino)-<br>''N''-(2,6-dimethylphenyl)acetamide | ||
| image = | | image = LidocaineStructure.png | ||
| width = 200px | | width = 200px | ||
<!--Clinical data--> | <!--Clinical data--> | ||
| Line 57: | Line 203: | ||
| melting_point = 68 | | melting_point = 68 | ||
}} | }} | ||
|mechAction=Studies of the effects of therapeutic concentrations of lidocaine on the electrophysiological properties of mammalian [[Purkinje fibers]] have shown that lidocaine attenuates phase 4 diastolic depolarization, decreases automaticity and causes a decrease or no change in excitability and membrane responsiveness. | |||
|structure=Lidocaine Hydrochloride Injection USP, is a sterile, aqueous solution of lidocaine, an antiarrhythmic agent, prepared with the aid of hydrochloric acid. It is intended for intravenous administration by either direct injection or continuous infusion. | |||
Lidocaine hydrochloride is designated 2-(Diethylamino)-2’, 6’-acetoxylidide monohydrochloride and isrepresented by the following structural formula: | |||
[[File:LidocaineStructure.png|600px|thumbnail|left|This image is provided by the National Library of Medicine.]] | |||
{{clr}} | |||
*pH of the above solution adjusted with sodium hydroxide and/or hydrochloric acid to finished product pH limits between 5 and 7. | |||
The medication and fluid pathway of these disposable syringes are sterile and nonpyrogenic in the original, unopened package with component caps in place. These dosage forms do not contain preservatives; once the unit is assembled and used, any remaining portion of the solution must be discarded with the entire unit. | |||
|PD=Action potential duration and effective refractory period of [[Purkinje fibers]] are decreased, while the ratio of effective refractory period to action potential duration is increased. Action potential duration and effective refractory period of ventricular muscle are also decreased. Effective refractory period of the [[AV node]] may increase, decrease or remain unchanged, and atrial effective refractory period is unchanged. Lidocaine raises the [[ventricular fibrillation]] threshold. No significant interactions between lidocaine and the autonomic nervous system have been described and consequently lidocaine has little or no effect on autonomic tone. | |||
Clinical electrophysiological studies with lidocaine have demonstrated no change in [[sinus node]] recovery time or sinoatrial conduction time. [[AV nodal conduction]] time is unchanged or shortened, and [[His-Purkinje conduction]] time is unchanged. | |||
=====Hemodynamics===== | |||
At therapeutic doses, lidocaine has minimal hemodynamic effects in normal subjects and in patients with heart disease. Lidocaine has been shown to cause no, or minimal, decrease in ventricular contractility, cardiac output, arterial pressure or heart rate. | |||
|PK=Lidocaine is rapidly metabolized by the liver, and less than 10% of a dose is excreted unchanged in the urine. Oxidative N dealkylation, a major pathway of metabolism, results in the metabolites monoethylglycinexylidide and glycinexylidide. The pharmacological/toxicological activities of these metabolites are similar to, but less potent than, lidocaine. The primary metabolite in urine is a conjugate of 4-hydroxy-2,6,-dimethylaniline. | |||
The elimination half-life of lidocaine following an intravenous bolus injection is typically 1.5 to 2 hours. There are data that indicate that the half-life may be 3 hours or longer following infusions of greater than 24 hours. | |||
Because of the rapid rate at which lidocaine is metabolized, any condition that alters liver function, including changes in liver blood flow, which could result from severe congestive heart failure in shock, may alter lidocaine kinetics. The half-life may be two-fold or more, greater in patients with liver dysfunction. [[Renal dysfunction]] does not affect lidocaine kinetics, but may increase the accumulation of metabolites. Therapeutic effects of lidocaine are generally associated with plasma levels at 6 to 25 μmole/L (1.5 to 6 mcg free base per mL). The blood to plasma distribution ratio is approximately 0.84. Objective adverse manifestations become increasingly apparent with increasing plasma levels above 6 mcg free base per mL. | |||
The plasma protein binding of lidocaine is dependent on drug concentration, and the fraction bound decreases with increasing concentration. At concentrations of 1 to 4 mcg free base per mL, 60 to 80 percent of lidocaine is protein bound. In addition to lidocaine concentration, the binding is dependent on the plasma concentration of the α-1-acid glycoprotein. | |||
Lidocaine readily crosses the placental and blood-brain barriers. [[Dialysis]] has negligible effects on the kinetics of lidocaine. | |||
|nonClinToxic=Long term studies in animals to evaluate the carcinogenic and mutagenic potential or the effect on fertility of lidocaine hydrochloride have not been conducted. | |||
|clinicalStudies=There is limited information regarding clinical studies testing the efficacy of lidocaine provided by the label. | |||
|howSupplied=In unit-use packages containing a Luer-JetTM Luer-Lock Prefilled Syringe, ten cartons per package: | |||
* Concentration: 2% | |||
* Stock No.: 3390 | |||
* NDC No.: 76329-3390-1 | |||
* Size: 5 mL (100 mg) | |||
|storage=* Store at 20 to 25°C (68 to 77°F). | |||
|fdaPatientInfo=The patients should be advised of the possible occurrence of the experiences listed under adverse reactions. | |||
|alcohol=Alcohol-Lidocaine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|brandNames= | |||
= | |||
* | |||
* | |||
* | |||
* | |||
|nlmPatientInfo=(Link to patient information page) | |||
|drugShortage=Drug Shortage | |||
}} | |||
{{LabelImage | |||
|fileName=LidocaineHydrochloridePackage1.png | |||
}} | |||
[[Category:Local anesthetics]] | |||
[[Category:Antiarrhythmic agents]] | [[Category:Antiarrhythmic agents]] | ||
[[Category:Cardiovascular Drugs]] | [[Category:Cardiovascular Drugs]] | ||
[[Category:Drug]] | [[Category:Drug]] | ||
Latest revision as of 16:36, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Lidocaine (injection) is a antiarrhythmic, local anesthetic that is FDA approved for the treatment of ventricular arrhythmias such as those occurring in relation to acute myocardial infarction, or during cardiac manipulation, such as cardiac surgery. Common adverse reactions include bradyarrhythmia, hypotension, backache dizziness, headache, lightheadedness, numbness, paresthesia, shivering, somnolence, blurred vision, burning sensation in eye, conjunctival hyperemia, corneal epithelial defect, diplopia, apprehension, confusion, euphoria, feeling nervous..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Single Direct Intravenous Injection (bolus)
- Only the 50 and 100 mg dosage sizes should be used for direct intravenous injection. The usual dose is 50 to 100 mg of lidocaine hydrochloride (0.70 to 1.4 mg/kg; 0.32 to 0.63 mg/lb) administered intravenously under ECG monitoring. This dose may be administered at the rate of approximately 25 to 50 mg/min (0.35 to 0.70 mg/kg/min; 0.16 to 0.32 mg/lb/min).
- Sufficient time should be allowed to enable a slow circulation to carry the drug to the site of action. If the initial injection of 50 to 100 mg does not produce a desired response, a second dose may be injected after 5 minutes. No more than 200 to 300 mg of lidocaine hydocloride should be administered during a one hour period.
Continuous Intravenous Infusion
- Following bolus administration, intravenous infusions of lidocaine hydrochloride may be initiated at the rate of 1 to 4 mg/min of lidocaine hydrochloride (0.014 to 0.057 mg/kg/min; 0.006 to 0.026 mg/lb/min). The rate of intravenous infusions should be reassessed as soon as the patient’s basic cardiac rhythm appears to be stable or at the earliest signs of toxicity. It should rarely be necessary to continue intravenous infusions of lidocaine for prolonged periods.
- Solutions for intravenous infusion may be prepared by the addition of one gram (or two grams) of lidocaine hydrochloride to one liter of 5% dextrose in water using aseptic technique. Approximately a 0.1% (or 0.2%) solution will result from this procedure; that is, each milliliter will contain approximately 1 (or 2) mg of lidocaine hydrochloride. In those cases in which fluid restriction is medically appropriate, a more concentrated solution may be prepared.
- Lidocaine hydrochloride injection has been found to be chemically stable for 24 hours after dilution in 5% dextrose in water. However, as with all intravenous admixtures, dilution of the solution should be made just prior to its administration.
- It is very important that after adding lidocaine hydrochloride, or any other medication, to an I.V. container, the contents be thoroughly mixed before beginning the infusion.
- When administering by continuous I.V. infusion, it is advisable to use a precision volume control I.V. set.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Lidocaine (injection) in adult patients.
Non–Guideline-Supported Use
Aortocoronary Bypass Grafting
- Dosing Information
Regional Pain Syndrome
- Dosing Information
- Lidocaine continuous infusion administered as a initial dose of 200 mg through the first hour, followed by 100 to 190 mg/h according to tolerance. Infusion should be continue until maximum pain control is reached.[3]
Infusion Pain
- Dosing Information
Cough
- Dosing Information
Elective Abortion
- Dosing Information
- 7 to 30 mL of 1% lidocaine administered through the umbilical vein, associated to 5 mcg of sufentanil, both administered 48 hours following mifepristone treatment (600 mg).[8]
Fibromyalgia
- Dosing Information
- Administer an initial dose of 5 mg/kg minus 100 mg, followed by 50 mg/day increases up to 5 mg/kg plus 150 mg. Do not excede 550 mg infused. Infusion should be administered over 6 hours, diluted in 500 mL of Hartman's solution.[9]
Indigestion
- Dosing Information
- 30 mL of antacid associated with 15 mL of 2% viscous lidocaine, both administered simultaneously PO.[10]
Peritubular Block
- Dosing Information
- 5 mL of lidocaine (2%) plus 5 mL bupivacaine 0.75% with 150 IU of hyaluronidase.[11]
Tumescent Anesthesia-Liposuction Procedure
- Dosing Information
- Administer a solution of: lidocaine 500 to 1000 mg + epinephrine 0.5 mg + sodium bicarbonate 10 mEq + triamcinolone 10 mg + 1 liter saline solution 0.09%. Solution should be administered directly into the subcutaneous tissue undergoing the procedure at a rate of 150 mL/hour. Administer over an average time of 90 to 120 minutes.[12]
Postoperative Pain
- Dosing Information
- Administer 1.5 mg/kg 30 minutes before surgery, followed by a 1.5 mg/kg/hour continuous infusion through the first hour following surgery.[13]
Seizure
- Dosing Information
- Administer 1.5 to 2 mg/kg IV infucion over 2 minutes. If seizure recurrence is observed dosage can be repeated.[14]
Tinnitus
- Dosing Information
- 1.5 mg/kg IV.[15]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Controlled clinical studies in the pediatric population to establish dosing schedules have not been conducted. The American Heart Association’s Standards and Guidelines recommends a bolus dose of 1 mg/kg, and an infusion rate of between 20 to 50 mcg/kg/min for prolonged therapy. When drug clearance is reduced, as in patients with shock, congestive heart failure or cardiac arrest, the infusion rate should not exceed 20 mcg/kg/min.
Note Regarding Prolonged Infusion: There are data that indicate the half-life may be 3 hours or longer following infusions of greater than 24 hours in duration. Note: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever the solution and container permit.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Lidocaine in pediatric patients.
Non–Guideline-Supported Use
Seizure
- Dosing Information
- Initial dose of 4 to 6 mg/kg/hr, followed by infusions of 4 to 8 mg/kg/hr administered over 11 to 60 hours until successful response was achieved.[16]
Contraindications
- Hypersensitivity to local anesthetics of the amide type.
- Stokes-Adams syndrome.
- Wolff-Parkinson-White syndrome.
- Patients with severe degrees of sinoatrial, atrioventricular, or intraventricular block in the absence of an artificial pacemaker.
Warnings
- Systemic toxicity may result in manifestations of central nervous system depression (sedation) or irritability (twitching), which may progress to frank convulsions accompanied by respiratory depression and/or arrest. Early recognition of premonitory signs, assurance of adequate oxygenation and, where necessary, establishment of artificial airway with ventilatory support are essential to management of this problem. Should convulsions persist despite ventilatory therapy with oxygen, small increments of anticonvulsant drugs may be used intravenously. Examples of such agents include benzodiazepines (e.g., diazepam), ultra short-acting barbiturates (e.g., thiopental or thiamylal), or a short-acting barbiturate (e.g., pentobarbital or secobarbital). If the patient is under anesthesia, a short-acting muscle relaxant (e.g., succinylcholine) may be used. Longer acting drugs should be used only when recurrent convulsions are evidenced.
- Should circulatory depression occur, vasopressors may be used.
- Constant electrocardiographic monitoring is essential to the proper administration of lidocaine hydrochloride. Signs of excessive depression of cardiac electrical activity such as sinus node dysfunction, prolongation of the P-R interval and QRS complex or the appearance or aggravation of arrhythmias, should be followed by flow adjustment and, if necessary, prompt cessation of the intravenous infusion of this agent. Occasionally, acceleration of ventricular rate may occur when lidocaine hydrochloride is administered to patients with atrial flutter or atrial fibrillation.
Precautions
General
- Caution should be employed in the use of lidocaine hydrochloride in patients with severe liver disease or kidney disease because accumulation of the drug or metabolites may occur.
- Lidocaine hydrochloride should be used with caution in the treatment of patients with hypovolemia, severe congestive heart failure, shock, and all forms of heart block. In patients with sinus bradycardia or incomplete heart block, the administration of lidocaine hydrochloride intravenously for the elimination of ventricular ectopic beats, without prior acceleration in heart rate (e.g., by atropine, isoproterenol or electric pacing), may promote more frequent and serious ventricular arrhythmias or complete heart block.
- Dosage should be reduced for children and for debilitated and/or elderly patients, commensurate with their age and physical status.
- The safety of amide local anesthetic agents in patients with genetic predisposition to malignant hyperthermia has not been fully assessed; therefore, lidocaine should be used with caution in such patients.
- In hospital environments where drugs known to be triggering agents for malignant hyperthermia (fulminant hypermetabolism) are administered, it is suggested that a standard protocol for management should be available.
- It is not known whether lidocaine may trigger this reaction; however, large doses resulting in significant plasma concentrations, as may be achieved by intravenous infusion, pose potential risk to these individuals. Recognition of early unexplained signs of tachycardia, tachypnea, labile blood pressure and metabolic acidosis may precede temperature elevation. Successful outcome is dependent on early diagnosis, prompt discontinuance of the triggering agent and institution of treatment including oxygen therapy, supportive measures and dantrolene.
Adverse Reactions
Clinical Trials Experience
Adverse experiences following the administration of lidocaine are similar in nature to those observed with other amide local anesthetic agents. Adverse experiences may result from high plasma levels caused by excessive dosage or may result from a hypersensitivity, idiosyncrasy or diminished tolerance on the part of the patient. Serious adverse experiences are generally systemic in nature. The following types are those most commonly reported. The adverse experiences under Central Nervous System and Cardiovascular System are listed, in general, in a progression from mild to severe.
Central Nervous System
CNS reactions are excitatory and/or depressant, and may be characterized by lightheadedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensations of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness, respiratory depression and respiratory arrest. The excitatory reactions may be very brief or may not occur at all, in which case, the first manifestation of toxicity may be drowsiness, merging into unconsciousness and respiratory arrest.
Cardiovascular System
Cardiovascular reactions are usually depressant in nature and are characterized by bradycardia, hypotension, and cardiovascular collapse, which may lead to cardiac arrest.
Allergic Reactions
Allergic reactions as a result of sensitivity to lidocaine are extremely rare and, if they occur, should be managed by conventional means.
Drug Abuse or Dependance
Although specific studies have not been conducted, lidocaine hydrochloride has been used clinically without evidence of abuse of this drug or of physiological or physical dependence as a result of its use.
Postmarketing Experience
There is limited information regarding Lidocaine (injection) Postmarketing Experience in the drug label.
Drug Interactions
- Lidocaine hydrochloride should be used with caution in patients with digitalis toxicity accompanied by atrioventricular block.
- Concomitant use of beta blockers may reduce hepatic blood flow and thereby reduce lidocaine clearance.
- Lidocaine and tocainide are pharmacologically similar.
- The concomitant use of these two agents may cause an increased incidence of adverse reactions, including central nervous system adverse reactions such as seizure.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
Reproduction studies have been performed in rats at doses up to 6.6 times the maximum human doses and have revealed no significant findings. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predicted of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Lidocaine (injection) in women who are pregnant.
Labor and Delivery
The effects of lidocaine hydrochloride on the mother and the fetus, when used in the management of cardiac arrhythmias during labor and delivery, are not known. Lidocaine readily crosses the placental barrier.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when lidocaine is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in children have not been established by controlled clinical studies.
Geriatic Use
There is no FDA guidance on the use of Lidocaine (injection) in geriatric settings.
Gender
There is no FDA guidance on the use of Lidocaine (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Lidocaine (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Lidocaine (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Lidocaine (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Lidocaine (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Lidocaine (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
Lidocaine hydrochloride is administered intravenously under ECG monitoring.
IV Compatibility
There is limited information regarding the IV compatibility provided by the label.
Overdosage
- Overdosage of lidocaine hydrochloride usually results in signs of central nervous system or cardiovascular system toxicity.
- Should convulsions or signs of respiratory depression and respiratory arrest develop, the patency of the airway and adequacy of ventilation must be assured immediately. Should convulsions persist despite ventilatory therapy with oxygen, small increments of anticonvulsive agents may be given intravenously. Examples of such agents include a benzodiazepine (e.g., diazepam), an ultrashort-acting barbiturate (e.g., thiopental or thiamylal), or a short-acting barbiturate (e.g., pentobarbital or secobarbital). If the patient is under general anesthesia, a short-acting muscle relaxant (e.g., succinylcholine) may be administered.
- Should circulatory depression occur, vasopressors may be used. Should cardiac arrest occur, standard CPR procedures should be instituted.
- Dialysis is of negligible value in the treatment of acute overdosage from lidocaine hydrochloride.
Pharmacology
Mechanism of Action
Studies of the effects of therapeutic concentrations of lidocaine on the electrophysiological properties of mammalian Purkinje fibers have shown that lidocaine attenuates phase 4 diastolic depolarization, decreases automaticity and causes a decrease or no change in excitability and membrane responsiveness.
Structure
Lidocaine Hydrochloride Injection USP, is a sterile, aqueous solution of lidocaine, an antiarrhythmic agent, prepared with the aid of hydrochloric acid. It is intended for intravenous administration by either direct injection or continuous infusion.
Lidocaine hydrochloride is designated 2-(Diethylamino)-2’, 6’-acetoxylidide monohydrochloride and isrepresented by the following structural formula:
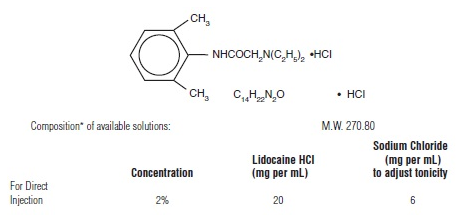
- pH of the above solution adjusted with sodium hydroxide and/or hydrochloric acid to finished product pH limits between 5 and 7.
The medication and fluid pathway of these disposable syringes are sterile and nonpyrogenic in the original, unopened package with component caps in place. These dosage forms do not contain preservatives; once the unit is assembled and used, any remaining portion of the solution must be discarded with the entire unit.
Pharmacodynamics
Action potential duration and effective refractory period of Purkinje fibers are decreased, while the ratio of effective refractory period to action potential duration is increased. Action potential duration and effective refractory period of ventricular muscle are also decreased. Effective refractory period of the AV node may increase, decrease or remain unchanged, and atrial effective refractory period is unchanged. Lidocaine raises the ventricular fibrillation threshold. No significant interactions between lidocaine and the autonomic nervous system have been described and consequently lidocaine has little or no effect on autonomic tone.
Clinical electrophysiological studies with lidocaine have demonstrated no change in sinus node recovery time or sinoatrial conduction time. AV nodal conduction time is unchanged or shortened, and His-Purkinje conduction time is unchanged.
Hemodynamics
At therapeutic doses, lidocaine has minimal hemodynamic effects in normal subjects and in patients with heart disease. Lidocaine has been shown to cause no, or minimal, decrease in ventricular contractility, cardiac output, arterial pressure or heart rate.
Pharmacokinetics
Lidocaine is rapidly metabolized by the liver, and less than 10% of a dose is excreted unchanged in the urine. Oxidative N dealkylation, a major pathway of metabolism, results in the metabolites monoethylglycinexylidide and glycinexylidide. The pharmacological/toxicological activities of these metabolites are similar to, but less potent than, lidocaine. The primary metabolite in urine is a conjugate of 4-hydroxy-2,6,-dimethylaniline.
The elimination half-life of lidocaine following an intravenous bolus injection is typically 1.5 to 2 hours. There are data that indicate that the half-life may be 3 hours or longer following infusions of greater than 24 hours.
Because of the rapid rate at which lidocaine is metabolized, any condition that alters liver function, including changes in liver blood flow, which could result from severe congestive heart failure in shock, may alter lidocaine kinetics. The half-life may be two-fold or more, greater in patients with liver dysfunction. Renal dysfunction does not affect lidocaine kinetics, but may increase the accumulation of metabolites. Therapeutic effects of lidocaine are generally associated with plasma levels at 6 to 25 μmole/L (1.5 to 6 mcg free base per mL). The blood to plasma distribution ratio is approximately 0.84. Objective adverse manifestations become increasingly apparent with increasing plasma levels above 6 mcg free base per mL.
The plasma protein binding of lidocaine is dependent on drug concentration, and the fraction bound decreases with increasing concentration. At concentrations of 1 to 4 mcg free base per mL, 60 to 80 percent of lidocaine is protein bound. In addition to lidocaine concentration, the binding is dependent on the plasma concentration of the α-1-acid glycoprotein.
Lidocaine readily crosses the placental and blood-brain barriers. Dialysis has negligible effects on the kinetics of lidocaine.
Nonclinical Toxicology
Long term studies in animals to evaluate the carcinogenic and mutagenic potential or the effect on fertility of lidocaine hydrochloride have not been conducted.
Clinical Studies
There is limited information regarding clinical studies testing the efficacy of lidocaine provided by the label.
How Supplied
In unit-use packages containing a Luer-JetTM Luer-Lock Prefilled Syringe, ten cartons per package:
- Concentration: 2%
- Stock No.: 3390
- NDC No.: 76329-3390-1
- Size: 5 mL (100 mg)
Storage
- Store at 20 to 25°C (68 to 77°F).
Images
Drug Images
{{#ask: Page Name::Lidocaine (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Lidocaine (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
The patients should be advised of the possible occurrence of the experiences listed under adverse reactions.
Precautions with Alcohol
Alcohol-Lidocaine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Lidocaine (injection) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Lidocaine (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Sunamori M, Okamura T, Amano J, Suma H, Suzuki A (1982). "Myocardial protection by lidocaine hydrochloride in aorto-coronary bypass surgery". Jpn J Surg. 12 (2): 93–7. PMID 6981016.
- ↑ Baraka A, Kawkabani N, Dabbous A, Nawfal M (2000). "Lidocaine for prevention of reperfusion ventricular fibrillation after release of aortic cross-clamping". J Cardiothorac Vasc Anesth. 14 (5): 531–3. doi:10.1053/jcan.2000.9484. PMID 11052433.
- ↑ Linchitz RM, Raheb JC (1999). "Subcutaneous infusion of lidocaine provides effective pain relief for CRPS patients". Clin J Pain. 15 (1): 67–72. PMID 10206569.
- ↑ Ho CM, Tsou MY, Sun MS, Chu CC, Lee TY (1999). "The optimal effective concentration of lidocaine to reduce pain on injection of propofol". J Clin Anesth. 11 (4): 296–300. PMID 10470630.
- ↑ Eriksson M, Englesson S, Niklasson F, Hartvig P (1997). "Effect of lignocaine and pH on propofol-induced pain". Br J Anaesth. 78 (5): 502–6. PMID 9175962.
- ↑ Gefke K, Andersen LW, Friesel E (1983). "Lidocaine given intravenously as a suppressant of cough and laryngospasm in connection with extubation after tonsillectomy". Acta Anaesthesiol Scand. 27 (2): 111–2. PMID 6837243.
- ↑ Stewart RH, Kimbrough RL, Engstrom PF, Cameron B (1988). "Lidocaine: an anti-tussive for ophthalmic surgery". Ophthalmic Surg. 19 (2): 130–1. PMID 3347458.
- ↑ Senat MV, Fischer C, Bernard JP, Ville Y (2003). "The use of lidocaine for fetocide in late termination of pregnancy". BJOG. 110 (3): 296–300. PMID 12628271.
- ↑ Raphael JH, Southall JL, Treharne GJ, Kitas GD (2002). "Efficacy and adverse effects of intravenous lignocaine therapy in fibromyalgia syndrome". BMC Musculoskelet Disord. 3: 21. PMC 126218. PMID 12217079.
- ↑ Welling LR, Watson WA (1990). "The emergency department treatment of dyspepsia with antacids and oral lidocaine". Ann Emerg Med. 19 (7): 785–8. PMID 2202240.
- ↑ Gao F, Budd AJ (1996). "Venous levels of lignocaine and bupivacaine after peribulbar block". Anaesthesia. 51 (12): 1109–12. PMID 9038442.
- ↑ Ostad A, Kageyama N, Moy RL (1996). "Tumescent anesthesia with a lidocaine dose of 55 mg/kg is safe for liposuction". Dermatol Surg. 22 (11): 921–7. PMID 9063507.
- ↑ Koppert W, Weigand M, Neumann F, Sittl R, Schuettler J, Schmelz M; et al. (2004). "Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery". Anesth Analg. 98 (4): 1050–5, table of contents. PMID 15041597.
- ↑ Pascual J, Sedano MJ, Polo JM, Berciano J (1988). "Intravenous lidocaine for status epilepticus". Epilepsia. 29 (5): 584–9. PMID 3409844.
- ↑ Israel JM, Connelly JS, McTigue ST, Brummett RE, Brown J (1982). "Lidocaine in the treatment of tinnitus aurium. A double-blind study". Arch Otolaryngol. 108 (8): 471–3. PMID 7049137.
- ↑ Lundqvist M, Ågren J, Hellström-Westas L, Flink R, Wickström R (2013). "Efficacy and safety of lidocaine for treatment of neonatal seizures". Acta Paediatr. 102 (9): 863–7. doi:10.1111/apa.12311. PMID 23738612.
{{#subobject:
|Label Page=Lidocaine (injection) |Label Name=LidocaineHydrochloridePackage1.png
}}