Melting point
The melting point of a solid is the temperature range at which it changes state from solid to liquid. Although the phrase would suggest a specific temperature and is commonly and incorrectly used as such in most textbooks and literature, most crystalline compounds actually melt over a range of a few degrees or less. At the melting point the solid and liquid phase exist in equilibrium. When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point. Because of the ability of some substances to supercool, the freezing point is not considered to be a characteristic property of a substance.
Fundamentals

For most substances, melting and freezing points are essentially equal. For example, the melting point and freezing point of the element mercury is 234.32 kelvin (−38.83 °C or −37.89 °F). However, certain substances possess differing solid-liquid transition temperatures. For example, agar melts at 85 °C (185 °F) and solidifies from 31 °C to 40 °C (89.6 °F to 104 °F); this process is known as hysteresis.
Certain materials, such as glass, may harden without crystallizing; these are called amorphous solids. Amorphous materials as well as some polymers do not have a true melting point as there is no abrupt phase change at any specific temperature. Instead, there is a gradual change in their viscoelastic properties over a range of temperatures. Such materials are characterized by a glass transition temperature which may be roughly defined as the "knee" point of the material's density vs. temperature graph.
The melting point of ice at 1 atmosphere of pressure is very close [1] to 0 °C (32 °F, 273.15 K), this is also known as the ice point. In the presence of nucleating substances the freezing point of water is the same as the melting point, but in the absence of nucleators water can supercool to −42 °C (−43.6 °F, 231 K) before freezing.
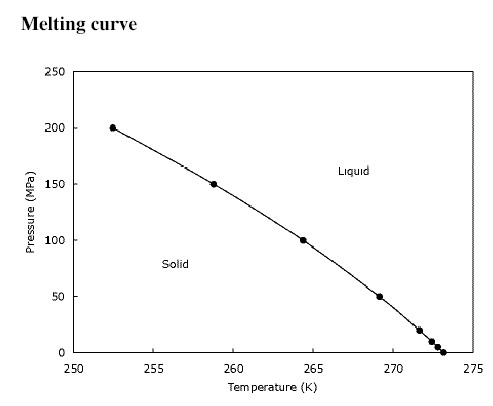
Unlike the boiling point, the melting point is relatively insensitive to pressure because the solid/liquid transition represents only a small change in volume.[2][3] Melting points are often used to characterize organic compounds and to ascertain the purity. The melting point of a pure substance is always higher and has a smaller range than the melting point of an impure substance. The more impurity is present, the lower the melting point and the broader the range. Eventually, a minimum melting point will be reached. The mixing ratio that results in the lowest possible melting point is known as the eutectic point.
The chemical element with the highest melting point is tungsten, at 3695 K (3422 °C, 6192 °F) making it excellent for use as filaments in light bulbs. The often-cited carbon does not melt at ambient pressure but sublimates at about 4000 K; a liquid phase only exists above pressures of 10 MPa and estimated 4300–4700 K. Tantalum hafnium carbide (Ta4HfC5) is a refractory compound with a very high melting point of 4488 K (4215 °C, 7619 °F).[4] At the other end of the scale, helium does not freeze at all at normal pressure, even at temperatures infinitesimally close to absolute zero; pressures over 20 times normal atmospheric pressure are necessary.
Melting point measurements
Many Laboratory techniques exist for the determination of melting points. A Kofler bench is a metal strip with a temperature gradient (range room temperature to 300°C). Any substance can be placed on a section of the strip revealing its thermal behaviour at the temperature at that point. Differential scanning calorimetry gives information on melting point together with its Enthalpy of fusion.
A basic melting point apparatus for the analysis of crystalline solids consists of a oil bath with a transparent window (most basic design: a Thiele tube) and a simple magnifier. The several grains of a solid are placed in a thin glass tube and partially immersed in the oil bath. The oil bath is heated (and stirred) and with the aid of the magnifier (and external light source) melting of the individual crystals at a certain temperature can be observed. In contemporary devices this optical detection is automated.
Thermodynamics
Not only is heat required to raise the temperature of the solid to the melting point, but the melting itself requires heat called the heat of fusion.
From a thermodynamics point of view, at the melting point the change in Gibbs free energy (<math>\Delta G</math>) of the material is zero, because the enthalpy (<math>H</math>) and the entropy (<math>S</math>) of the material are increasing (<math>\Delta H, \Delta S > 0</math>). Melting phenomenon happens when the Gibbs free energy of the liquid becomes lower than the solid for that material. At various pressures this happens at a specific temperature. It can also be shown that:
<math>\Delta S = \frac {\Delta H} {T}</math>
The "<math>T</math>","<math>\Delta S</math>", and "<math>\Delta H</math>" in the above are respectively the temperature at the melting point, change of entropy of melting, and the change of enthalpy of melting.
Carnelley’s Rule
In organic chemistry Carnelley’s Rule established in 1882 by Thomas Carnelley, states that high molecular symmetry is associated with high melting point [5]. Carnelley based his rule on examination of 15,000 chemical compounds. For example for three structural isomers with molecular formula C5H12 the melting point increases in the series isopentane −160 °C (113 K) n-pentane −129.8 °C (143 K) and neopentane −18 °C (255 K). Likewise in xylenes and also dichlorobenzenes the melting point increases in the order meta, ortho and then para. Pyridine has a lower symmetry than benzene hence its lower melting point but the melting point again increases with diazine and triazines. Many cage-like compounds like adamantane and cubane with high symmetry have very high melting points.
A high melting point results from a high heat of fusion or a low entropy of fusion or a combination of both. In highly symmetrical molecules the crystal phase is densely packed with many efficient intermolecular interactions resulting in a higher enthalpy change on melting.
See also
- Boiling point
- Freezing-point depression
- List of elements by melting point
- Melting
- Melting Points for various elements
- Phases of matter
- Triple point
- Vicat softening point - the determination of the softening point for materials that have no definite melting point.
References
- ↑ The melting point of purified water has been measured to be 0.002519 +/- 0.000002 degrees Celsius - see R. Feistel and W. Wagner (2006). "A New Equation of State for H2O Ice Ih". J. Phys. Chem. Ref. Data. 35: 1021–1047.
- ↑ The exact relationship is expressed in the Clausius-Clapeyron relation.
- ↑ "J10 Heat: Change of aggregate state of substances through change of heat content: Change of aggregate state of substances and the equation of Clapeyron-Clausius". Retrieved 2008-02-19.
- ↑ hafnium entry at Britannica.com
- ↑ Melting Point and Molecular Symmetry R. J. C. Brown, R. F. C. Brown Journal of Chemical Education 724 Vol. 77 No. 6 June 2000
External links
- Melting and boiling point tables vol. 1 by Thomas Carnelley (Harrison, London, 1885-1887)
- Melting and boiling point tables vol. 2 by Thomas Carnelley (Harrison, London, 1885-1887)
af:Smeltpunt ar:نقطة انصهار ast:Puntu de fusión bs:Talište bg:Температура на топене ca:Punt de fusió cs:Teplota tání da:Smeltepunkt de:Schmelzpunkt et:Sulamistemperatuur el:Σημείο τήξης eo:Frostopunkto eu:Urtze-puntu fa:دمای ذوب gl:Punto de fusión ko:녹는점 hr:Talište id:Titik lebur is:Bræðslumark it:Punto di fusione he:נקודת התכה jv:Titik lebur sw:Kiwango cha kuyeyuka lv:Kušanas temperatūra lt:Lydymosi temperatūra jbo:selrunme lmo:Pünt de füsiun hu:Olvadáspont mk:Точка на топење ml:ദ്രവണാങ്കം ms:Takat lebur nl:Smeltpunt no:Smeltepunkt nn:Smeltepunkt uz:Erish harorati qu:Puriqchana iñu simple:Melting point sk:Teplota topenia sl:Tališče sr:Тачка топљења sh:Talište fi:Sulamispiste sv:Smältpunkt th:จุดหลอมเหลว uk:Температура плавлення zh-yue:冰點