Hypoplastic left heart syndrome
| Hypoplastic left heart syndrome | ||
 | ||
|---|---|---|
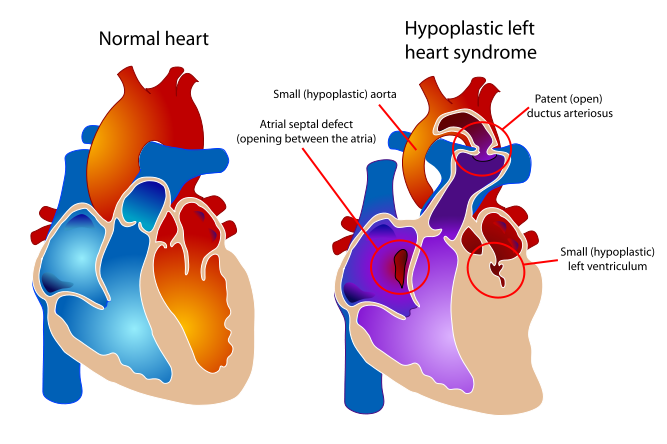
| Diagram of a healthy heart and one suffering from Hypoplastic left heart syndrome | ||
| ICD-10 | Q23.4 | |
| ICD-9 | 746.7 | |
| OMIM | 241550 | |
| DiseasesDB | 31507 | |
| MedlinePlus | 001106 | |
| eMedicine | ped/1131 | |
| MeSH | C14.240.400.625 | |
Template:Search infobox Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editors-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]; Keri Shafer, M.D. [3]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [4] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Hypoplastic left heart syndrome (also known as HLHS), is a rare congenital heart defect in which the left side of the heart is severely underdeveloped. In babies with HLHS, the aorta and left ventricle are very small, and the aortic and mitral valves are either too small to allow sufficient blood flow or are atretic (closed) altogether. As blood returns from the lungs to the left atrium, it must pass through an atrial septal defect to the right side of the heart. [1] [2] [3] [4][5] [6] [7] [8] [9] [10] [11] [12] [13]
Etiology
While many authorities believe the cause of HLHS is unknown,[14] [15][16] recent research indicates that HLHS may be due to genetic factors. [17][18]
There is evidence associating it with Gap junction protein, alpha 1.[19]
Associated cardiac abnormalities
- Anomalous pulmonary venous connection
- Coarctation of the aorta
- Complete atrioventricular canal
- Coronary artery abnormalities (especially in patients with aortic atresia and mitral stenosis)
- Persistent left superior vena cava
- Endocardial fibroelastosis (especially in patients with aortic atresia and mitral stenosis)
Clinical Presentation
In babies with HLHS, the aorta and left ventricle are very small, and the aortic and mitral valves are either too small to allow sufficient blood flow or are atretic (closed) altogether. As blood returns from the lungs to the left atrium, it must pass through an atrial septal defect to the right side of the heart.
In a healthy human, the left side of the heart receives oxygen-rich blood from the lungs and pumps it out to the rest of the body; with these structures underdeveloped, they cannot circulate blood to other organs, and the right ventricle must pump blood to both the lungs, as it would normally, and to the rest of the body, a situation which cannot be sustained for long.
In cases of HLHS, the right side of the heart often must pump blood to the body through a patent ductus arteriosus. As the ductus arteriosus usually closes within eleven days after birth, blood flow is severely restricted and eventually cut off, leading to dangerously low circulation and eventually to shock.
Symptoms
- Bluish (cyanosis) or poor skin color
- Cold extremities
- Hepatomegaly
- Lethargy
- Poor pulse
- Poor suckling and feeding
- Pounding heart
- Rapid breathing
- Shortness of breath
- Sudden death
Differential Diagnosis
- Valvular aortic stenosis
- Viral myocarditis
- Unbalanced atrioventricular septal defect
- Total anomalous pulmonary venous connection
- Cardiac tumors
- Coarctation of the aorta
- Interrupted aortic arch
Diagnosis
- ECG shows enlargement of the right ventricle
- X-ray of the chest may show signs of other birth defects
- Echocardiogram
- Cardiac catheterization
Treatment
Without treatment, HLHS is fatal, but with intervention, an infant may survive. A pediatric cardiothoracic surgeon may perform a series of operations or a full heart transplant. In the meantime, the ductus may be kept open to allow bloodflow using medication containing prostaglandin. Because these operations are complex and need to be individualized for each patient, a cardiologist must assess all medical and surgical options on a case-by-case basis. After assessment the child's parents are given a percentage chance of survival and the choice of surgery or to take the child home to pass away.
The two methods for treatment of HLHS are transplantation and a three-stage surgical procedure. Each of these are open heart surgeries (meaning the chest is open and the sternum has to be separated), requiring the child to be on a heart bypass machine for at least five hours. The heart is stopped and the heart bypass machine pumps blood to the body. After successful surgery is performed there can be added complications of restarting the heart and getting the child off the heart bypass machine.
The three-stage procedure is a palliative procedure (not a cure), as the child's circulation is made to work with only two of the heart's four chambers.
- The first step is the Norwood procedure. In this procedure, the right ventricle is used to pump blood into the systemic circulation. Since the right ventricle is no longer directly pumping blood to the lungs, a shunt is required in order to pass deoxygenated blood through the lungs. Either the subclavian artery can be connected to the pulmonary circulation (Blalock-Taussig shunt), or a shunt is made directly from the right ventricle to the pulmonary circulation (Sano shunt). The narrow aorta is enlarged using a patch to improve bloodflow to the body.
During this time the baby may be medically fragile and have feeding problems because the heart is working very hard. There is a considerable degree of venous mixing in the right ventricle, leading to lower oxygenation saturations. In addition, the Blalock-Taussig shunt and the Sano shunt both expose the lungs to systemic arterial pressures, leading in the long term to pulmonary hypertension and eventually to heart failure.
- The second stage, the bi-directional Glenn procedure or Hemi-Fontan (see also Kawashima procedure) relieves some of the above problems. In this operation, the superior vena cava is ligated from the heart and connected to the pulmonary circulation. At this time, the Blalock-Taussig or Sano shunt is taken down. At this point, the lungs are no longer exposed to systemic arterial pressures, but much lower venous pressures. Although venous blood from the upper half of the body is no longer mixing with oxygenated blood in the right ventricle, there is still venous mixing from the lower half of the body, leading to some degree of oxygen desaturation.
During this time the child may have improved quality of life as the heart does not have to work as hard.
- The final procedure, the Fontan (Fontan procedure) completes the repair of the hypoplastic left heart. Although there are several variations, the functional effect is to redirect venous blood from the lower body (through the inferior vena cava) away from the right atrium to the pulmonary artery. Now, there should not be any mixing of oxygenated and deoxygenated blood in the right ventricle. The right ventricle performs the traditional job of the left, supplying the body with oxygenated blood, while the passive systemic venous pressure performs the traditional job of the right, passing deoxygenated blood to the lungs.
The Norwood Procedure is generally performed within a week of birth, the second stage at 3–6 months of age, and the Fontan at 18 months to four years of age. There are two types of Fontan: the Lateral Tunnel Fontan, and the Extracardiac Fontan. When the Fontan Procedure was first being done for children with HLHS, the only Fontan was the Lateral Tunnel Fontan. This requires actual cutting in the heart itself to create a "tunnel" by which the blood can travel passively to the lungs. Within the last decade, doctors have created an Extracardiac Fontan. This operation creates a tunnel outside the heart itself which reduces the chances of Fontan patients developing scar tissue on the heart which might later cause arrythmias.
Complications
- Irregular, fast heart rhythms (arrhythmias)
- Chronic diarrhea (due to protein loosing enteropathy)
- Heart failure
- Ascites and pleural effusion
- Blockage of the artificial shunt
- Strokes and other neurological complications
- Sudden death
Prognosis
While infants successfully treated for HLHS have a good chance of survival, they may experience chronic health problems for the rest of their lives. The 3-stage surgeries were developed in the early 1980's with no survivors prior to that time. Therefore, the earliest survivors are in their early 20's and the long term prognosis is unknown. However, the advances in surgical and medical techniques have helped increase the survival rate dramatically since the surgeries were first developed.
As is true for patients with other types of heart defects involving malformed valves [5], HLHS patients run a high risk of endocarditis, and must be monitored by a cardiologist for the rest of their lives to check on their heart function.
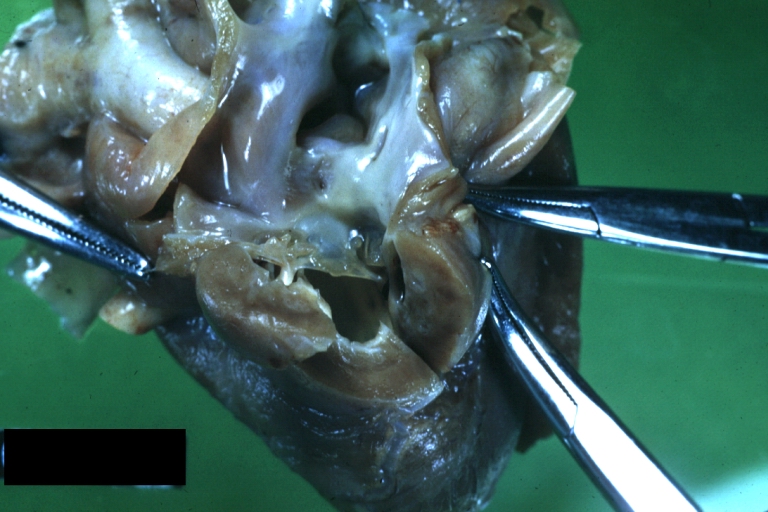
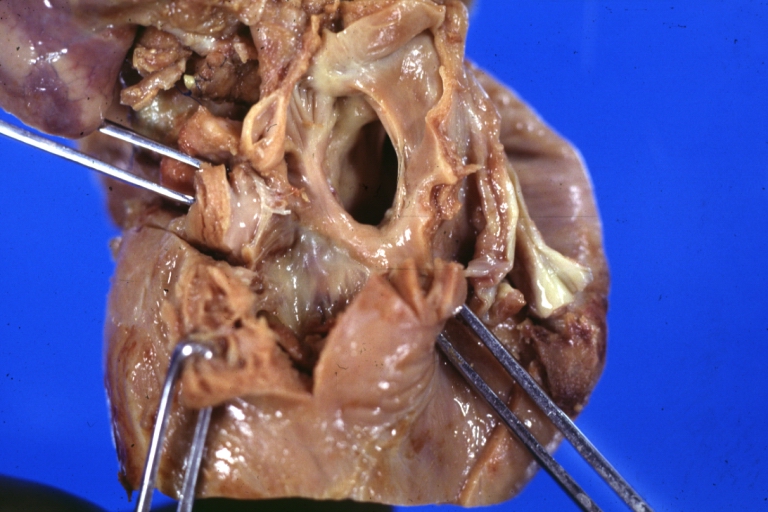
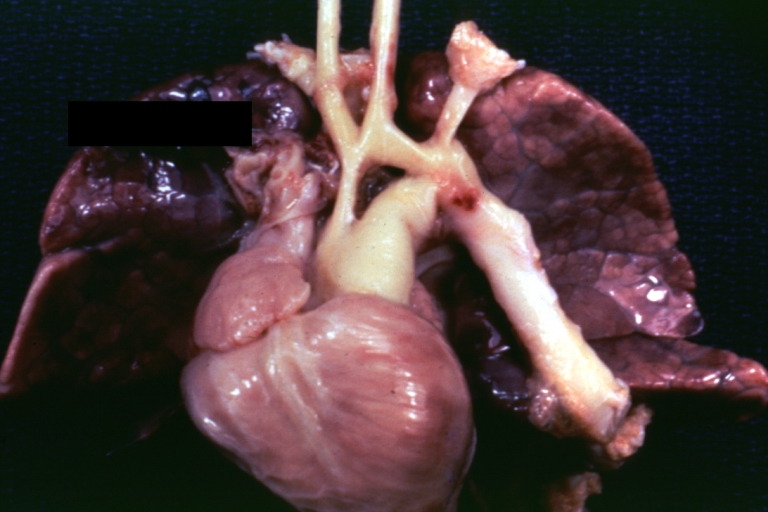
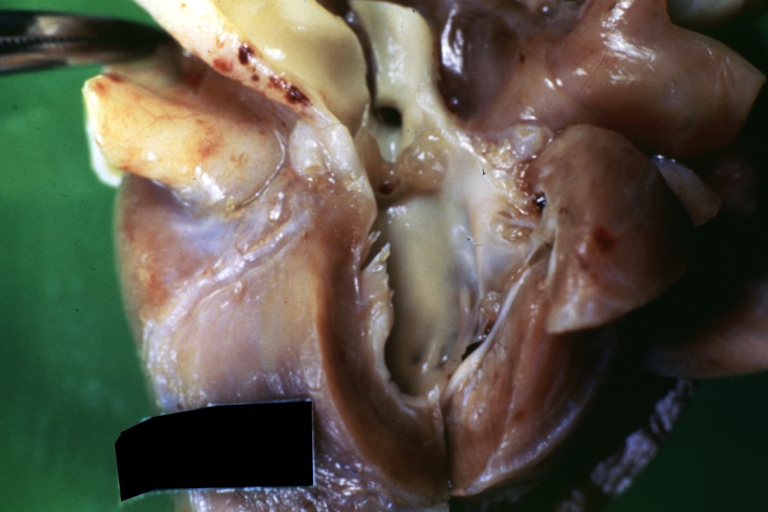
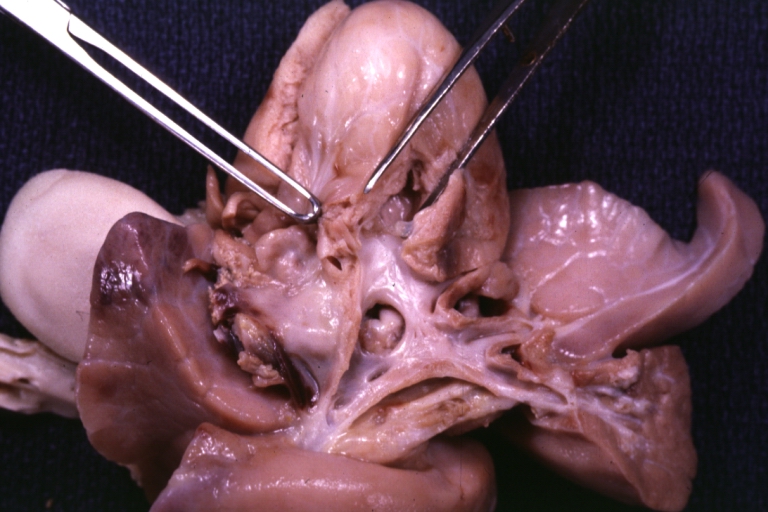
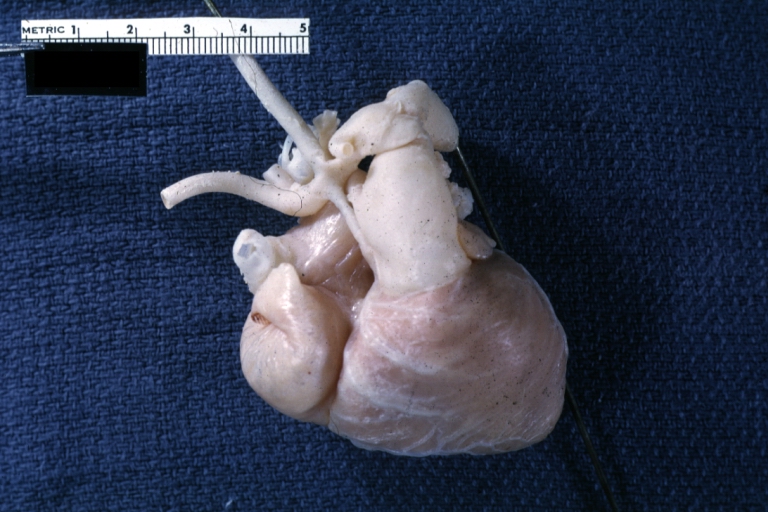
Pathological Findings
External links
- HLHS Information Page - created for parents expecting a child with HLHS. Includes questions to ask the physicians and insurance company.
- California Heart Connection - support network for those with congenital heart defects including Hypoplastic Left Heart Syndrome (HLHS.) Free online support group
- Hypoplastic Left Heart Syndrome information from Seattle Children's Hospital Heart Center
- Card-AG, The Cardiologycal Working Group of the University Pediatric Clinic Munster
- Hypoplastic Left Heart Syndrome, American Heart Association
- Overview at Cincinnati Children's Hospital Medical Center
- Overview at University of Michigan Congenital Heart Center
- Cardiac Center at The Children's Hospital of Philadelphia
- Little Hears Matter, a charity providing support and information on HLHS and other single ventricle heart defects
- Baby Hearts Press
- www.congenitalheartdefects.com
- 120+ stories of children born with Hypoplastic Left Heart Syndrome hosted by the The Congenital Heart Information Network.
- HLHS email support groups/discussion list hosted by the The Congenital Heart Information Network (membership required).
- Little Hearts, Inc. - Providing hope with stories of children with HLHS and other CHDs. Email support group (membership required)
- HeartLine, a charity for children with heart conditions and their families
Sources
- Card-AG, The Cardiologycal Working Group of the University Pediatric Clinic Munster
- Hypoplastic Left Heart Syndrome, American Heart Association
- Information on HLHS from the Cincinnati Children's Hospital Medical Center, accessed 9 July, 2006
- Hypoplastic Left Heart Syndrome - University of Michigan Congenital Heart Center
Template:SIB de:Hypoplastisches Linksherz-Syndrom nn:Hypoplastisk venstre hjarte-syndrom uk:Синдром гіпоплазії лівих відділів серця
- ↑ Atz AM, Feinstein JA, Jonas RA, et al. Preoperative management of pulmonary venous hypertension in hypoplastic left heart syndrome with restrictive atrial septal defect. Am J Cardiol. Apr 15 1999;83(8):1224-8.
- ↑ Backer CL, Bove EL, Zales VR. Hypoplastic left heart syndrome. In: Cardiac Surgery. New York, NY: Churchill Livingstone;1994:442-53.
- ↑ Bailey L, Concepcion W, Shattuck H, Huang L. Method of heart transplantation for treatment of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. Jul 1986;92(1):1-5.
- ↑ Barber G. Hypoplastic left heart syndrome. In: Garson A Jr, Bricker JT, Fisher DJ, Neish SR, eds. The Science and Practice of Pediatric Cardiology. 2nd ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 1998: 1625-45.
- ↑ Bove EL, Lloyd TR. Staged reconstruction for hypoplastic left heart syndrome. Contemporary results. Ann Surg. Sep 1996;224(3):387-94; discussion 394-5.
- ↑ Bove EL. Current status of staged reconstruction for hypoplastic left heart syndrome. Pediatr Cardiol. Jul-Aug 1998;19(4):308-15.
- ↑ Day RW, Barton AJ, Pysher TJ, Shaddy RE. Pulmonary vascular resistance of children treated with nitrogen during early infancy. Ann Thorac Surg. May 1998;65(5):1400-4.
- ↑ Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. May 1971;26(3):240-8.
- ↑ Freedom RM, Benson LN. Hypoplastic left heart syndrome. In: Moss and Adams Heart Disease in Infants, Children, and Adolescents. 5th ed. 1995: 1133-1153.
- ↑ Norwood WI, Kirklin JK, Sanders SP. Hypoplastic left heart syndrome: experience with palliative surgery. Am J Cardiol. Jan 1980;45(1):87-91.
- ↑ Norwood WI, Lang P, Hansen DD. Physiologic repair of aortic atresia-hypoplastic left heart syndrome. N Engl J Med. Jan 6 1983;308(1):23-6.
- ↑ Pizarro C, Malec E, Maher KO, et al. Right ventricle to pulmonary artery conduit improves outcome after stage I Norwood for hypoplastic left heart syndrome. Circulation. Sep 9 2003;108(10 Suppl 1):II155-II160.
- ↑ Talner CN. Report of the New England Regional Infant Cardiac Program, by Donald C. Fyler, MD, Pediatrics, 1980; 65(suppl):375-461. Pediatrics. Jul 1998;102(1 Pt 2):258-9.
- ↑ "Hypoplastic Left Heart Syndrome Causes - Mayo Clinic". Retrieved 2008-01-09.
- ↑ Hypoplastic Left Heart Syndrome : Article by P Syamasundar Rao, MD at eMedicine
- ↑ MedlinePlus Encyclopedia Hypoplastic left heart
- ↑ "Hypoplastic left heart syndrome likely caused by genetic factors". Retrieved 2008-01-09.
- ↑ Dasgupta C, Martinez AM, Zuppan CW, Shah MM, Bailey LL, Fletcher WH (2001). "Identification of connexin43 (alpha1) gap junction gene mutations in patients with hypoplastic left heart syndrome by denaturing gradient gel electrophoresis (DGGE)". Mutat. Res. 479 (1–2): 173–86. doi:10.1016/S0027-5107(01)00160-9. PMID 11470490.
- ↑ Online Mendelian Inheritance in Man (OMIM) HYPOPLASTIC LEFT HEART SYNDROME -241550