Dilated cardiomyopathy
| Dilated cardiomyopathy | |
 | |
|---|---|
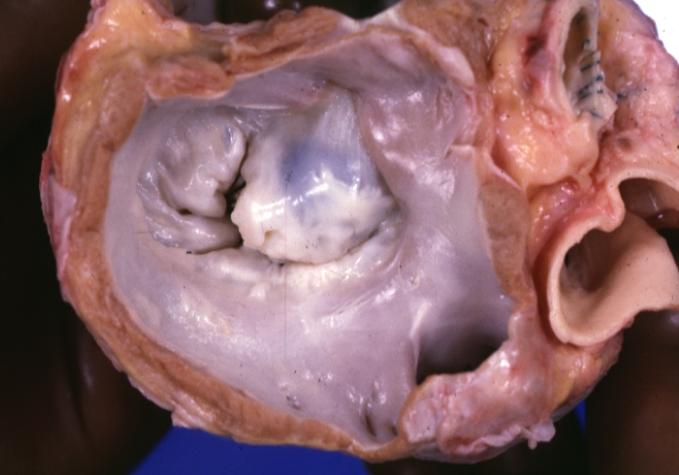
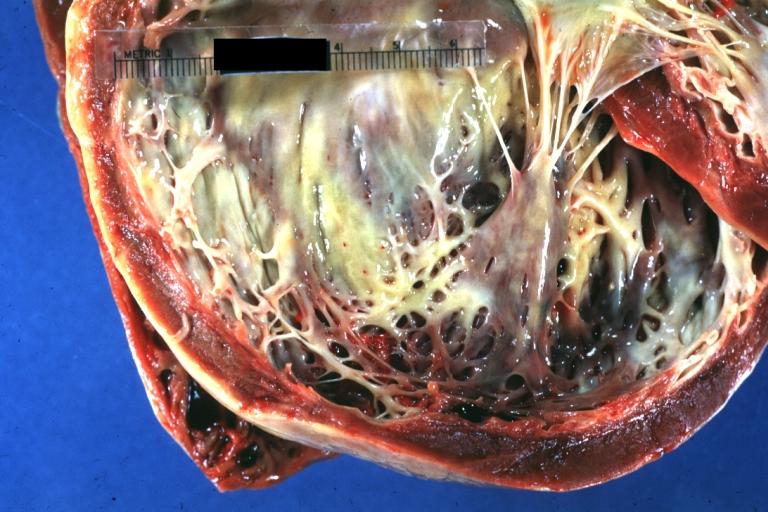
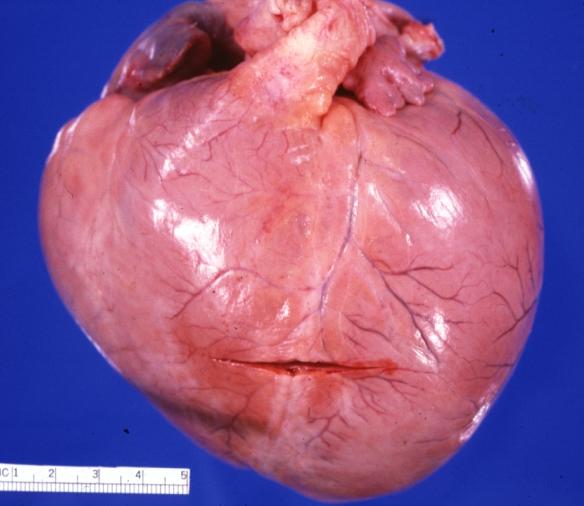
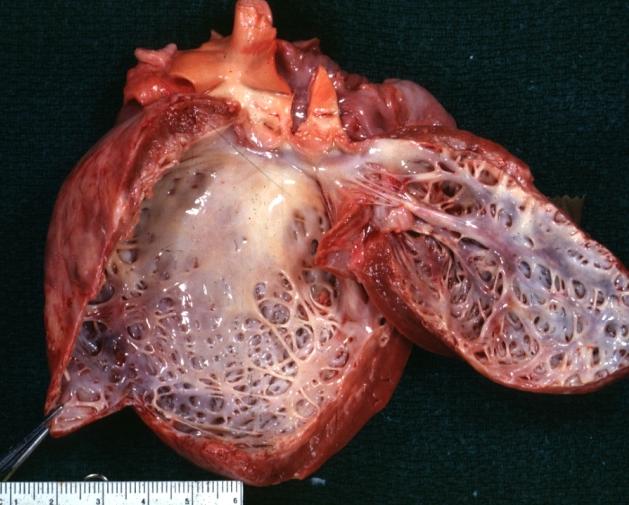
| Dilated Cardiomyopathy: Gross dilated left ventricle with marked endocardial sclerosis Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology | |
| ICD-10 | I42.0 |
| ICD-9 | 425.4 |
| OMIM | 212110 |
| DiseasesDB | 3066 |
| MedlinePlus | 000168 |
| eMedicine | med/289 emerg/80 ped/2502 |
| MeSH | D002311 |
| Cardiology Network |
 Discuss Dilated cardiomyopathy further in the WikiDoc Cardiology Network |
| Adult Congenital |
|---|
| Biomarkers |
| Cardiac Rehabilitation |
| Congestive Heart Failure |
| CT Angiography |
| Echocardiography |
| Electrophysiology |
| Cardiology General |
| Genetics |
| Health Economics |
| Hypertension |
| Interventional Cardiology |
| MRI |
| Nuclear Cardiology |
| Peripheral Arterial Disease |
| Prevention |
| Public Policy |
| Pulmonary Embolism |
| Stable Angina |
| Valvular Heart Disease |
| Vascular Medicine |
Editor-in-Chief: Sachin Shah, M.D.
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Dilated cardiomyopathy or DCM is a condition of the heart that causes dilation and impaired contraction of the left ventricle (or both ventricles). Impaired contraction is defined as a low ejection fraction (< 40%). In the following text the clinical presentation, diagnosis, and treatment will be reviewed.
Cardimyopathy as a general topic also includes topics such as restrictive cardiomyopathy and hypertrophic cardiomyopathy. This section will focus on dilated cardiomyopathy. There is sometimes confusion regarding nomenclature, some of this confusion is based on different classifications set forth by the WHO (World Health Organization) and the AHA (American Heart Association). The WHO classifies cardiomyopathy as either Dilated (DCM), Restrictive (RCM), or Hypertrophic (HCM). The American Heart Association classifies cardiomyopathies as either "primary" or "secondary."
Dilated cardiomyopathy can occur at any age (although it is more likely between the ages of 20-60)[1] there is a male predominance (3:1 male:female),[2], and is 2.5 times more likely in African Americans.[3]. There are many causes and there are varying degrees of severityof the disease . Some forms are reversible and some are irreversible; some patients may be completely asymptoamtic and some may require cardiac transplantation.
Causes
There are many causes of dilated cardiomyopathy. The most common cause is "idiopathic" which accounts for roughly 50% of cases.[4] The next most common is Myocarditis which accounts for roughly 10%. Ischemic "cardiomyopathy," infiltrative disease, hypertensive heart disease, substance abuse (i.e. alcohol abuse or cocaine abuse), connective tissue disease, peripartum cardiomyopathy, drugs (such as the chemotherapeutic agent doxarubacin), HIV infection or antiretroviral drugs, toxins (such as cobalt, lead or beryllium), and nutritional deficiencies (such as thiamine or selenium) are among other causes of dilated cardiomyopathy.
The high percentage of idiopathic dilated cardiomyopathy may be related to the difficulty in diagnosing viral myocarditis. There are not definitive diagnostic criteria for myocarditis based on echocardiography, the clinical presentation is commonly similar to dilated cardiomyopathy from other causes, and the sensitivity of endomycardial biopsy is relatively low and approaches near 50% even with immunostaining (CD3 for T lymphocytes and CD68 for macrophages).[5]. Cardiac MRI (Magnetic Resonance Imaging) appears to be not only helpful in the localization of inflammation and targeting of endomyocardial biopsy but also seems to be helpful in the diagnosis of myocarditis.[6] This new technology may be helpful in reducing the percentage of idiopathic dilated cardiomyopathy and discovering an etiology to the dilated cardiomyopathy in some of these patients.
Clinical Presentation
The clinical presentation of dilated cardiomyopathy is similar to that heart failure from any cause. Dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, lower extremity edema and orthostasis / syncope are all common findings in dilated cardiomyopathy. In addition dilated cardiomyopathy may present as palpitations as a result of arrhythmia (ventricular or atrial) with the most common arrhythmia being atrial fibrillation. Dilated cardiomyopathy may also present as sudden cardiac death or as CVA (cerebrovascular accident) or other embolic phenomenon (either from associated atrial fibrillation or from ventricular thrombi as a result of dilated ventricular cavities).
Angina is not a common feature of dilated cardiomyopathy unless the cause is related to coronary artery disease. If angina is present a work up for cardiac ischemia should be undertaken.[7]
Diagnosis
The diagnosis of dilated cardiomyopathy is based on clinical presentation and imaging findings. The most common imaging modality used to diagnose dilated cardiomyopathy is 2D-echocardiography. Echocardiographic findings of dilated cardiomyopathy include dilation of the left ventricle; however, may include dilation of all 4 cardiac chambers, LV (left ventricular) wall thickness usually is normal but given the dilation the LV mass is increased. In addition there is a global reduciton in systolic function. Occasionally there may also be wall motion abnormalities even in patients without flow limiting coronary artery disease.[8]
The diagnosis requires a dilated left ventricle and low ejection fraction.
In terms of determining the etiology a careful history is most instrumental. If the patient has CAD (coronary artery disease) risk factors, known CAD, or angina then a workup for CAD should be undertaken with coronary angiography. A viral prodrome such as viral URI or viral gastroenteritis may make viral myocarditis as a more likely cause. If the patient was exposed to chemotherapy such as anthracyclines then this would be the likely cause. Patients at risk for HIV should undergo testing as HIV can cause a dilated cardiomyopathy. Peripartum cardiomyopathy most often occurs within 1 month of delivery or 5 months after delivery, so recent childbirth is important information. Often by 8 months gestational age pregnancy is physically apparent but it is important to rule out pregnancy in women of childbearing age with dilated cardiomyopathy. Screening questions regarding cocaine or alcohol abuse or other toxin exposure (such as cobalt) should be addressed.
A review of systems is also helpful in regards to connective tissue disease associated dilated cardiomyopathy (which can be related to SLE (systemic lupus erythematosis), rheumatoid arthritis, sarcoidosis, scleroderma, as well as other connective tissue diseases).
A family history also has a great importance in the diagnosis of dilated cardiomyopathy. It has been suggested that a portion of those patients labeled as "idiopathic" may have a familial form of the disease. The prevalence of this in the population of patients with dilated cardiomyopathy has been estimated as high as 25%.[9] The majority of these are thought to be related to autosomal dominant transmission, the remaining are thought to be transimtted in an autosomal recessive and X-linked fashion.[10] Mitochondrial inheritance of the disease has also been identified.[11]
Pericardial effusion may accompany myocarditis but this finding is not specific. Cardiac MRI as discussed above may be helpful in diagnosing myocarditis. Endomyocardial biopsy as discussed above has low sensitivy and the findings are also notoriously non-specific. The findings on biopsy usually involve findings of inflammation and specific pathogens are unlikely to be identified. There may be an increased yield to using MRI to target endomyocardial biopsy as described above. Viral titiers (serologies) are often unhelpful and not routinely ordered in clinical practice.
Prognosis
There are many prognostic factors which can be evaluated in a patient with dilated cardiomyopathy.[12] The most important prognostic indicator is a decreased ejection fraction, in addition increased ventricular size and right ventricular dilation are independent indicators of a poor prognosis.
As is in most cases of heart failure a poor NYHA functional class and increased PASP (>35mmHg) are also poor prognostic indicators.
Other findings that infer a poor prognosis are as follows: Maximal O2 uptake of < 12mL/kg / minute on exercise testing, LBBB (left bundle branch block), non sustained ventricular tachycardia, syncope, hyponatremia with a serum sodium less than 135, elevated norepinephrine, ANP (atrial natriuretic peptide) and renin levels (not routinely measured in clinical practice), elevated PCWP (pulmonary capillary wedge pressure) > 18mmHg, low cardiac index < 2.5L/min/m^2.
Treatment
Death from dilated cardiomyopathy is usually the result of progressive heart failure or arrhythmia. Treatments for dilated cardiomyopathy are targeted towards preventing death and treating symptoms of heart failure. Some medications (such as ACE inhibitors) may help acheive both goals. Initial treatment should focus on the underlying cause of dilated cardiomyopathy if reversible. If for example a patient has dilated cardiomyopathy and three vessel coronary artery disease then revascularization should be an initial step in treatment.
Medications which have a proven survival benefit in systolic heart failure are ACE (angiotensin converting enzyme) inhibitors, ARBs (angiotensin II receptor blockers), beta blockers (carvedilol and metoprolol succinate are the most well studied), aldosterone antagonists (in severe heart failure) and combination of long acting nitrates and hydralazine. Medications targeted towards symptom relief in systolic heart failure are diuretics and digoxin. Warfarin is also a medication used in systolic heart failure; the benefit of this medication is controversial.
ACE inhibitors are thought to be useful in dilated cardiomyopathy by not only causing arterial vasodilation and reduction of afterload but also by reduction in levels of angiotensin II, aldosterone, and ADH which may have deleterious remodeling effects on the heart as well as vasoconstriction. ACE inhibitors have been extensively studied in systolic heart failure and have been shown to reduce symptoms, slow the progression of heart failure and reduce mortality in all classes of heart failure. The most profound impact is seen in patients who are most symptomatic as well as those patients who have recently suffered a myocardial infarction. In addition ACE inhibitors have been shown to slow the progression of LV (left ventricular) dilation in systolic heart failure.
The SOLVD-Prevention trial and SAVE trial both are large randomized controlled trials which evaluated patients with asymptomatic systolic heart failure. SOLVD-Prevention examined 4000 patients with an EF < 35% who were asymptomatic and randomized them to enalapril (dose 2.5-20mg daily) or placebo. After an average 3 years follow up there was a significant reduction in heart failure hospitalizations but no difference in mortality. The SAVE (Survival and Ventricular Enlargement) trial studied 2200 patients with an EF < or = to 40% within 3-14 days following an acute myocardial infarction. These patients were given either captopril or placebo and followed for an average of 3.5 years. There was a significant 25% reduciton in myocardial infarction and a significant reduction in death from recurrent MI by 32% in the captopril group.[13]
The CONSENSUS, SOLVD-Treatment and V-HeFT II trials all examined ACE inhibitors in symptomatic patients with systolic heart failure. The CONSENSUS trial was published in 1987 and studied 250 patients with NYHA class IV heart failure. These patients were randomized to enalapril (dose 2.5mg - 40mg daily) or placebo. After an average follow up of 6 months there was a 40% reduction in mortality in the enalapril group (this persisted to 1 year, at 1 year there was a 31% reduction in mortality in the enalapril group). The reduction in mortality was attributed to a reduction in the death from progressive heart failure, not from a reduction in sudden cardiac death. SOLVD-Treatement and V-HeFT II also showed a reduction in mortality with ACE inhibitors, both of these studies evalauted mainly patients with NYHA class II or III heart failure.[14][15]
The AIRE and TRACE trials were placebo controlled randomized controlled trials which both evaluated ACE inhibitors (ramipril and trandolapril respectively) in heart failure following a myocardial infarction. Both of these studies also showed a reduction in mortality in the ACE inhibitor arm when compared to placebo. These studies evaluated patients at a minimum of 2 (AIRE) or 3 (TRACE) days following an acute MI.
The CONSENSUS II, ISIS-4, GISSI-3 and SMILE studies all evaluated patients early (within 24 hours) following an acute myocardial infarction. All but the CONSENSUS II study showed a mortality benefit (both short and long term) in the ACE inhibitor arm when compared to placebo.[16][17][18]
ARBs have also been extensively studied. There are at least four large trials which support the use of ARBs in systolic heart failure. These include the ELITE, ELITE II, Val-HeFT, and CHARM trials. The ELITE trial randomized 722 patients over the age of 65 with an EF < 40% and NYHA class II-IV heart failure to either an ACEi (captopril) or an ARB (losartan). After a follow up of 48 weeks there was no difference in the primary endpoint (persistent elevations in serum creatinine) however a secondary endpoint of mortality showed a risk reduction of 46% in teh losartan group.[19] This was further evaluated in the ELITE II trial which randomized 3150 patients similar to those in the ELITE trial and again compared captopril and losartan. After a mean follow up of 555 days there was no difference in mortalitity or resuscitated arrest, but losartan was "better tolerated" than captopril (captopril was most often discontinued due to ACEi related cough).[20] The Val-HeFT trial was a placebo controlled RCT that evaluated studied valsartan use in 5000 patients with heart failure. There was a reduction in the combined endpoint of mortality and morbidity after a mean follow up of 23 months.[21]
CHARM randomized 76000 patients to candesartan or placebo. There were 3 subgroups analyzed in this study. CHARM Added showed that there was a reduction in cardiovascular mortality or CHF hospitlization in patients with systolic heart failure (EF < 40%) who were taking candesartan as well as an ACEi when compared to an ACEi alone. The CHARM Alternative subgroup showed that in ACEi intolerant patients with systolic heart failure (EF < 40%) there was a reduction in mortality or CHF hospitalization in patients taking an ARB (candesartan) when compared to placebo. The CHARM Preserved group evaluated patients with heart failure and a preserved ejection fraction and found no difference between candesartan and placebo.[22]
The data is very strongly in favor of using ACEi as first line medications for systolic heart failure, and the addition of ARBs may be helpful. In addition, if patients are intolerant of ACEi there are clear benefits to using ARBs.
Beta Blockers Aldosterone antagonists Long acting nitrate and hydralazine combinations
As stated above warfarin is a medication that is occasionally used in systolic heart failure and the benefits are controversial. In systolic heart failure and atrial fibrillation there is a more clear benefit in terms of stroke prevention; however, in isolated systolic heart failure the reduction in embolic risk has not been fully elucidated.
Loop diuretic medications are commonly used for patients with symptoms of heart failure. There has been no clear survival benefit associated with loop diuretics; however, the use of diuretics and a sodium restricted diet are the mainstays of treatment for congestive symptoms.
Digoxin is sometimes used in systolic heart failure for symptom relief. A survival benefit has not been shown with digoxin use; however, it is useful for symptomatic treatment. There may be more of a role for digoxin in systolic heart failure and atrial fibrillation as digoxin may serve a dual purpose of heart failure symptom relief related to increased ionotropy as well as ventricular rate control related to AV nodal blockade.
N-3 polyunsaturated fatty acids have also been evaluated by the GISSI-HF group and showed a slight reduction in mortality (from 29% to 27%) at roughly 3 years of follow up.[23]
Advanced Therapies
In patients with end stage heart failure or hemodynamic compromise vasopressor agents may be helpful. In patients with hemodynamic compromise medications such as dobutamine or dopamine may provide blood pressure support necessary. In addition parental agents such as milrinone (a phosphodiesterase inhibitor- somewhat specific for cardiac and vascular tissue) may be helpful in the short term or for end stage patients. There is no clear survival benefit and most trials with IV phosphodiesterase inhibitors are small trials of end stage heart failure patients. Oral milrinone has been studied and has been shown to increase mortality.
In patients who have severe heart failure and are refractory to medical therapy cardiac transplantation may be an option (based upon patient characteristics such as age and comorbidities as well as disease characterisitics such as the etiology of dilated cardiomyopathy). As a bridge to cardiac transplantation multiple mechanical devices have been used. The most durable device is the LVAD (left ventricular assist device), certain patient populations not suitable for cardiac transplantation may also undergo LVAD placement by a cardiac surgeon as a "destination LVAD." This may be considered a palliative measure for end-stage heart failure patients. Other devices which may be more limited in the duration of use include the aortic balloon pump, Impella, and the PVAD (peripheral ventricular assist device) one of which is the Tandem Heart.
Implantable cardiac defibrillators have also been extensively studied in systolic heart failure. A survival benefit has also been shown in select patient populations.
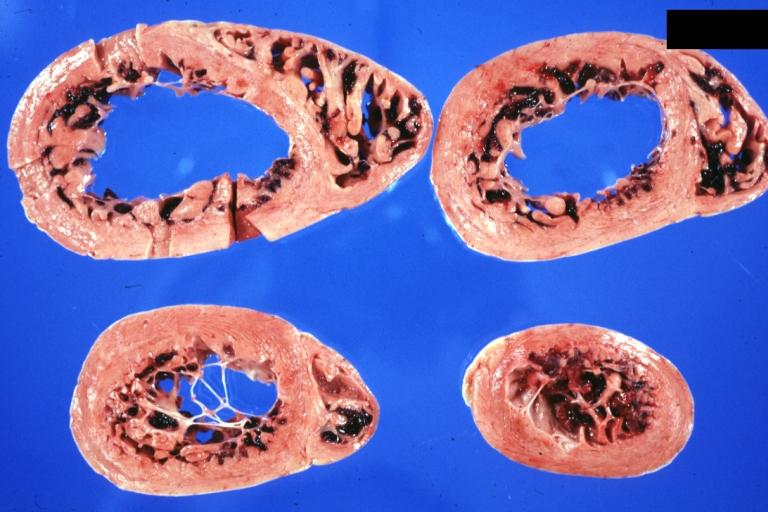
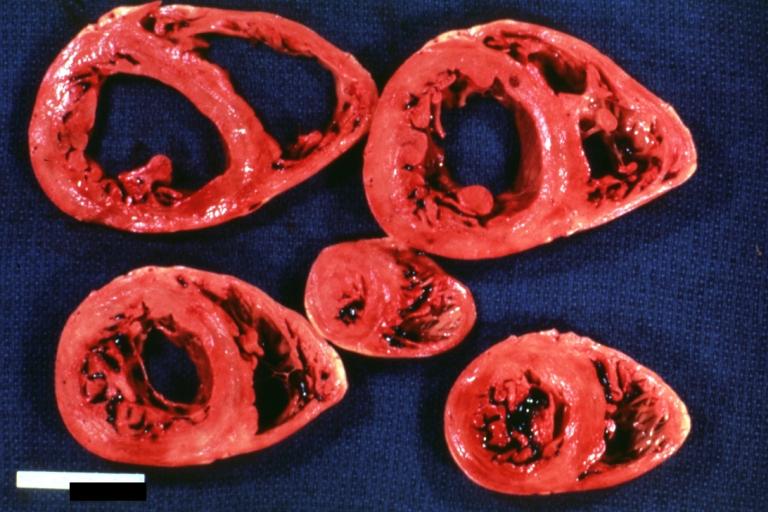
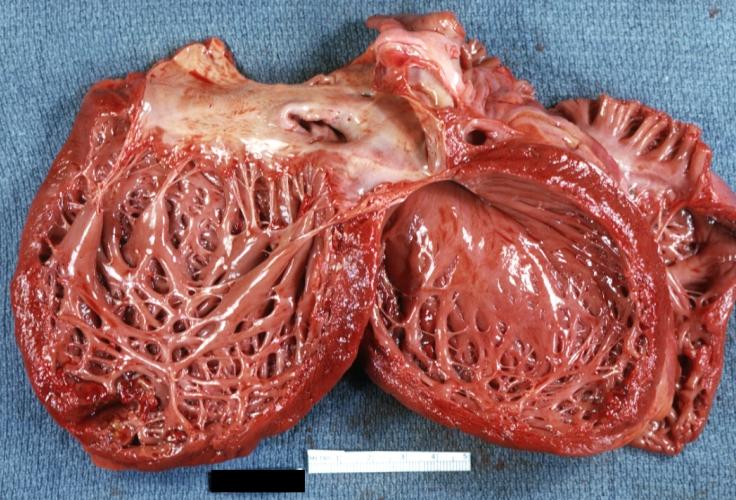
Gross Pathological Findings
Images shown below are Courtesy of Professor Peter Anderson DVM PhD and published with permission. © PEIR, University of Alabama at Birmingham, Department of Pathology
-
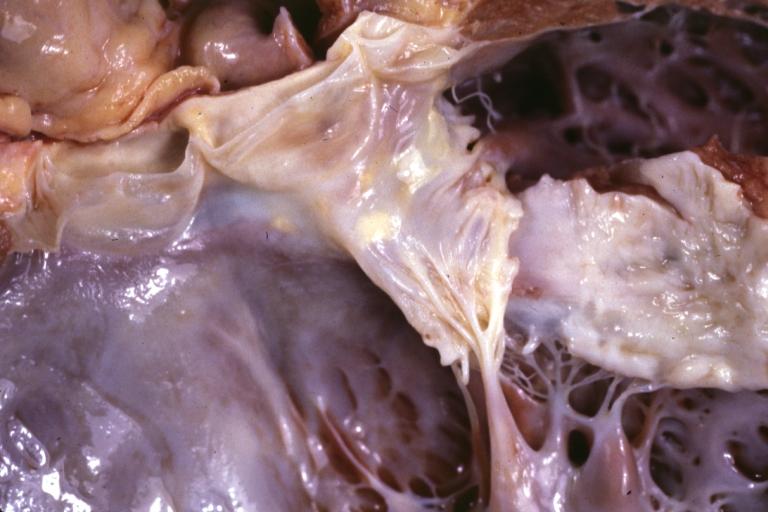
Cardiomyopathy: Gross excellent view of mitral valve from left atrium anterior leaflet appears to balloon a bit into the atrium
-
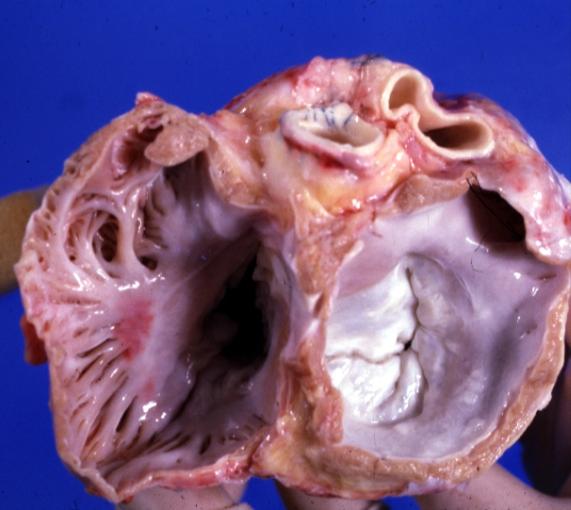
Cardiomyopathy: Gross excellent view of mitral and tricuspid valves from atria, appear normal anatomy.
-
Dilated Cardiomyopathy: Gross natural color close-up view of heart surgically removed for a transplantation shows aortic valve and anterior leaflet of mitral valve with cholesterol deposits endocardium of left ventricle is diffusely thickened
-
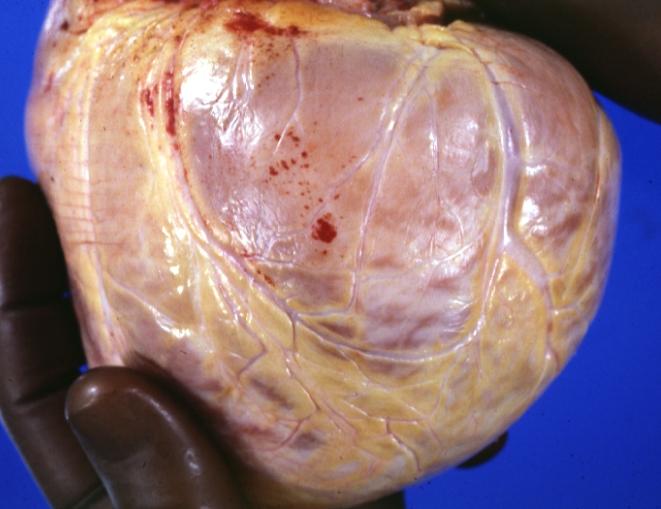
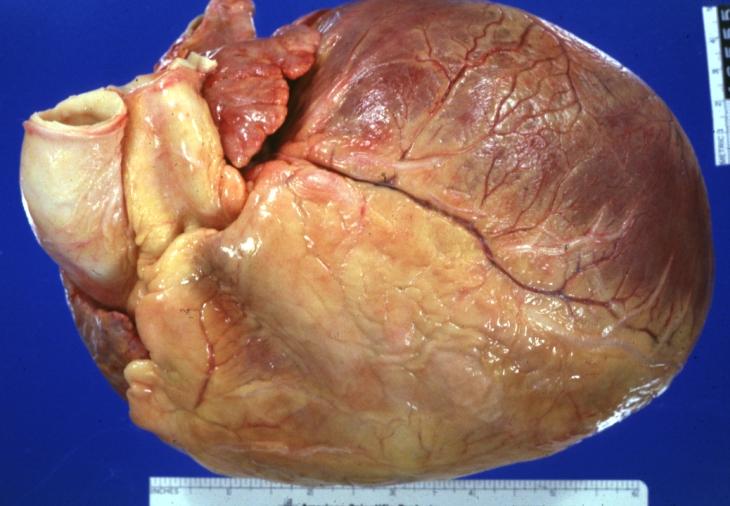
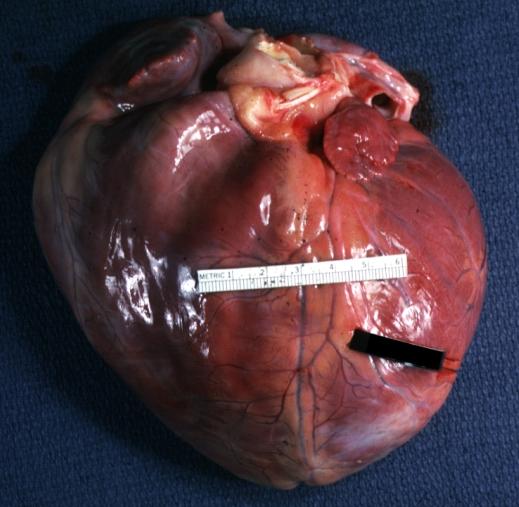
Cardiomyopathy: Gross external view of globular heart with patchy fibrosis seen through epicardium
-
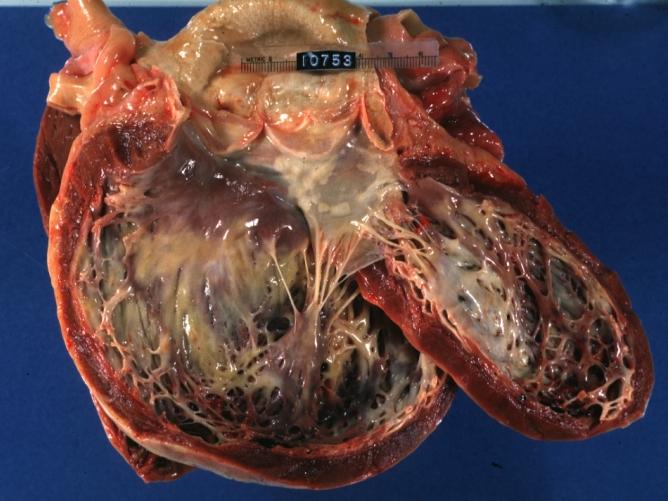
Cardiomyopathy: Gross dilated left ventricle with marked endocardial thickening this is what has been called adult fibroelastosis
-
Dilated Cardiomyopathy: Gross good example huge dilated left ventricle
-
Dilated Cardiomyopathy: Gross dilated left ventricle with marked endocardial sclerosis (an excellent example)
-
Cardiomyopathy: Gross intact globular shaped heart
-
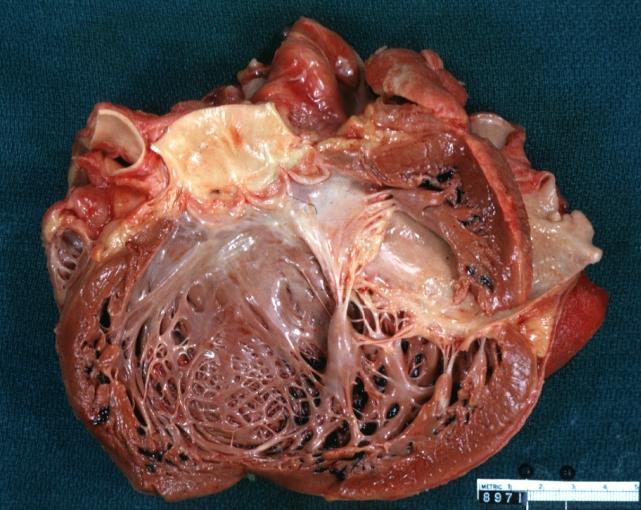
Dilated Cardiomyopathy: Gross opened left ventricle dilated with endocardial thickening good example
-
Cardiomyopathy: Gross globular heart external view 10 year old girl with sickle cell anemia
-
Cardiomyopathy: Gross horizontal sections of ventricles dilation type 10 year old girl with sickle cell anemia
-
Cardiomyopathy: Intermediate between hypertrophic and dilated
-
Dilated Cardiomyopathy: Gross opened globular left ventricle natural color (very good example)
-
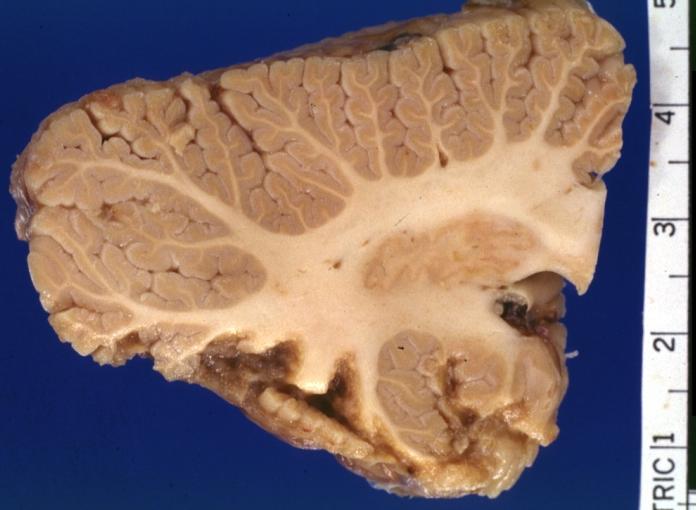
Brain: Infarct: Healing large MCA and PICA probably embolic 64 year old female chronic obstructive pulmonary disease and cardiomyopathy with atrial fibrillation
-
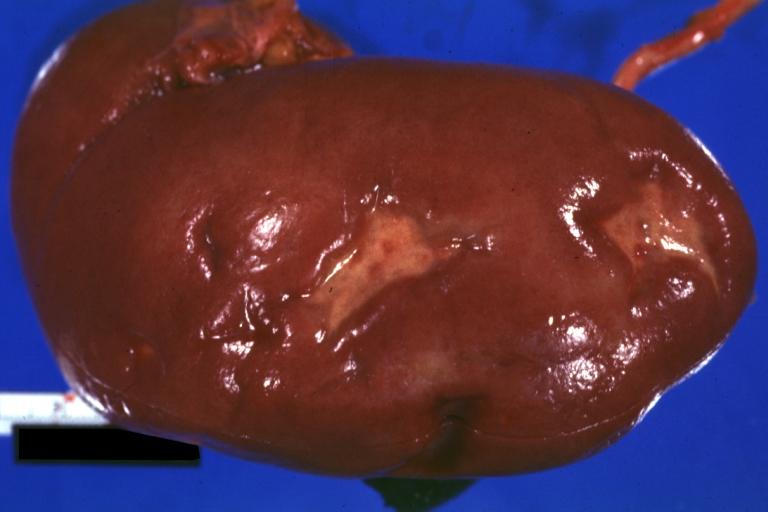
Kidney: Infarct Remote: Gross external view with capsule removed two old and very typical infarct scars 27yobf with dilated cardiomyopathy
-
Dilated Cardiomyopathy: Gross natural color external view globular heart 500 gm 24yo female seven pregnancies
References
- ↑ Dec GW, Fuster V. Idiopathic Dilated Cardiomyopathy. N Engl J Med 1994 Dec 8;331(23):1564-75. PMID 7969328
- ↑ Robbins Basic Pathology, 7th edition. Kumar, Cotran, Robbins. ISBN 0-7216-9274-5
- ↑ Coughlin SS, Labenberg JR, Tefft MC. Black-white differences in idiopathic dilated cardiomyopathy: the Washington DC Dilated Cardiomyopathy Study. Epidemiology. 1993;4:165-72. PMID 8452906
- ↑ Felker GM, Thompson RE, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000 Apr 13;342(14):1077-84.
- ↑ Herskowitz A, Ahmed-Ansari A, Neumann DA et al. Induction of major histocompatibility complex antigens within the myocardium of patients with active myocarditis: a nonhistologic marker of myocarditis. J Am Coll Cardiol. 1990;15(3):624-632.
- ↑ Freidrich MG, Strohm O, et al. Contrast media-enhanced mangetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97(18):1802-1809.
- ↑ Mayo Clinic Cardiology. Concise Textbook. Murphy, Joseph G; Lloyd, Margaret A. Mayo Clinic Scientific Press. 2007.
- ↑ Mayo Clinic Cardiology. Concise Textbook. Murphy, Joseph G; Lloyd, Margaret A. Mayo Clinic Scientific Press. 2007.
- ↑ Ross J Jr. Dilated cardiomyopathy: concepts derived from gene deficient and transgenic animal models. Circ J. 2002;66:219-24. PMID 11922267
- ↑ Mestroni L; Rocco C; et al. Familial dilated cardiomyopathy: evidence for genetic and phenotypic heterogeneity. Heart Muscle Disease Study Group. J Am Coll Cardiol 1999 Jul;34(1):181-90.
- ↑ Schonberger J, Seidman CE. Many roads lead to a broken heart: the genetics of dilated cardiomyopathy. Am J Hum Genet. 2001;69:249-60. Epub 2001 Jul 6. PMID 11443548
- ↑ Mayo Clinic Cardiology. Concise Textbook. Murphy, Joseph G; Lloyd, Margaret A. Mayo Clinic Scientific Press. 2007.
- ↑ Circulation, Oct 1994;90:1731-1738.
- ↑ N Engl J Med. 1991 Aug 1;325(5):303-10
- ↑ N Engl J Med. 1991 Aug 1;325(5):293-302.
- ↑ Lancet. 1995 Mar 18;345(8951):669-85
- ↑ Lancet. 1994 May 7;343(8906):1115-22
- ↑ N Engl J Med. 1995 Jan 12;332(2):80-5.
- ↑ Lancet 1997;349:747-52
- ↑ Lancet. 2000;355:1582-1587.
- ↑ N Engl J Med. 2001 Dec 6;345(23):1667-75
- ↑ Circulation 2004; 110:2180
- ↑ Tavazzi L; Maggioni AP, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008 Oct 4;372(9645):1223-30. Epub 2008 Aug 29.
See Also
External links
Additional Reading
- Moss and Adams' Heart Disease in Infants, Children, and Adolescents Hugh D. Allen, Arthur J. Moss, David J. Driscoll, Forrest H. Adams, Timothy F. Feltes, Robert E. Shaddy, 2007 ISBN 0781786843
- Braunwald's Heart Disease, Libby P, 8th ed., 2007, ISBN 978-1-41-604105-4
- Hurst's the Heart, Fuster V, 12th ed. 2008, ISBN 978-0-07-149928-6
- Willerson JT, Cardiovascular Medicine, 3rd ed., 2007, ISBN 978-1-84628-188-4