Sandbox Abciximab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Sandbox Abciximab is a Platelet aggregation inhibitor that is FDA approved for the {{{indicationType}}} of prevention of cardiac ischemic complications in patients undergoing percutaneous coronary intervention and in patients with unstable angina not responding to conventional medical therapy when percutaneous coronary intervention is planned within 24 hours. Common adverse reactions include Chest pain , Hypotension ,Injection site pain ,Abdominal pain , Nausea, Vomiting Bleeding Backache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Preparation of PCI
- Dosing Information
- initial dosage:0.25 mg/kg IV bolus (10-60 minutes before the start of PCI)
- maitaining dosage: 0.125 mg/kg/min IV for 12 hours (max 10 ug/min)
Treatment of patient with unstable angina planned to undergo PCI within 24 hours
- Dosing Information
- initial dosage:0.25 mg/kg IV (10-60 minutes before the start of PCI)
- maitaining dosage: 10 ug/min IV for 18- 24h(concluding one hour after the PCI)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Myocardial ischemia
- Class of Recommendation: Class IIa
- Level of Evidence: Level A
- Recommendation
- initial dosage: 0.25 mg/kg IV bolus (over 5 minutes)
- maitaing dosage: 10 ug/min IV (continued for 12 hours unless complications developed)
Non–Guideline-Supported Use
Acute myocardial infarction
- Dosing information
- initial dosage: 0.25 mg/kg IV bolus
- maitaing dosage: 10 ug/min IV for 12h [1]
Acute arterial thrombosis
- Dosing information
- initial dosage: 0.25 mg/kg IV bolus
- maitaing dosage: 10 ug/min IV for 12h [2]
Cerebrovascular accident
- Dosing information
- initial dosage: 0.25 mg/kg IV bolus
- maitaing dosage: 10 ug/min IV for 12h [3]
Myocardial infarction
- Dosing information
- initial dosage: 0.25 mg/kg IV bolus
- maitaing dosage: 10 ug/min IV for 12h [4]
Prophylaxis of myocardial ischemia
- Dosing information
- initial dosage: 0.25 mg/kg IV bolus
- maitaing dosage: 10 ug/min IV for 12h
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Sandbox Abciximab FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Non–Guideline-Supported Use
Aneurysm of coronary vessels
- Dosing information
- loading dose: 0.25 mg/kg IV
- follow-up: 0.125 mg/min IV for 12h [5]
Contraindications
Because Abciximab may increase the risk of bleeding, Abciximab is contraindicated in the following clinical situations:
- Active internal bleeding
- Recent (within six weeks) gastrointestinal (GI) or genitourinary (GU) bleeding of clinical significance.
- History of cerebrovascular accident (CVA) within two years, or CVA with a significant residual neurological deficit
- Bleeding diathesis
- Administration of oral anticoagulants within seven days unless prothrombin time is <1.2 times control
- Thrombocytopenia (<100,000 cells/mL)
- Recent (within six weeks) major surgery or trauma
- Intracranial neoplasm, arteriovenous malformation, or aneurysm
- Severe uncontrolled hypertension
- Presumed or documented history of vasculitis
- Use of intravenous dextran before PCI, or intent to use it during an intervention
Abciximab is also contraindicated in patients with known hypersensitivity to any component of this product or to murine proteins.
Warnings
Bleeding Events
Abciximab has the potential to increase the risk of bleeding events, rarely including those with a fatal outcome, particularly in the presence of anticoagulation, e.g., from heparin, other anticoagulants, or thrombolytics (see ADVERSE REACTIONS: Bleeding).
The risk of major bleeds due to Abciximab therapy is increased in patients receiving thrombolytics and should be weighed against the anticipated benefits.
Should serious bleeding occur that is not controllable with pressure, the infusion of Abciximab and any concomitant heparin should be stopped.
Allergic Reactions (including anaphylaxis)
Allergic reactions, some of which were anaphylaxis (sometimes fatal), have been reported rarely in patients treated with ReoPro. Patients with allergic reactions should receive appropriate treatment. Treatment of anaphylaxis should include immediate discontinuation of ReoPro administration and initiation of resuscitative measures.
Precautions
Bleeding Precautions
To minimize the risk of bleeding with Abciximab, it is important to use a low-dose, weight-adjusted heparin regimen, a weight-adjusted Abciximab bolus and infusion, strict anticoagulation guidelines, careful vascular access site management, discontinuation of heparin after the procedure and early femoral arterial sheath removal.
Therapy with Abciximab requires careful attention to all potential bleeding sites including catheter insertion sites, arterial and venous puncture sites, cutdown sites, needle puncture sites, and gastrointestinal, genitourinary, pulmonary (alveolar), and retroperitoneal sites.
Arterial and venous punctures, intramuscular injections, and use of urinary catheters, nasotracheal intubation, nasogastric tubes and automatic blood pressure cuffs should be minimized. When obtaining intravenous access, non-compressible sites (e.g., subclavian or jugular veins) should be avoided. Saline or heparin locks should be considered for blood drawing. Vascular puncture sites should be documented and monitored. Gentle care should be provided when removing dressings.
Femoral artery access site
Arterial access site care is important to prevent bleeding. Care should be taken when attempting vascular access that only the anterior wall of the femoral artery is punctured, avoiding a Seldinger (through and through) technique for obtaining sheath access. Femoral vein sheath placement should be avoided unless needed. While the vascular sheath is in place, patients should be maintained on complete bed rest with the head of the bed ≤ 30° and the affected limb restrained in a straight position. Patients may be medicated for back/groin pain as necessary.
Discontinuation of heparin immediately upon completion of the procedure and removal of the arterial sheath within six hours is strongly recommended if APTT ≤ 50 sec or ACT≤ 175 sec (see PRECAUTIONS: Laboratory Tests). In all circumstances, heparin should be discontinued at least two hours prior to arterial sheath removal.
Following sheath removal, pressure should be applied to the femoral artery for at least 30 minutes using either manual compression or a mechanical device for hemostasis. A pressure dressing should be applied following hemostasis. The patient should be maintained on bed rest for six to eight hours following sheath removal or discontinuation of Abciximab, or four hours following discontinuation of heparin, whichever is later. The pressure dressing should be removed prior to ambulation. The sheath insertion site and distal pulses of affected leg(s) should be frequently checked while the femoral artery sheath is in place and for six hours after femoral artery sheath removal. Any hematoma should be measured and monitored for enlargement.
The following conditions have been associated with an increased risk of bleeding and may be additive with the effect of Abciximab in the angioplasty setting: PCI within 12 hours of the onset of symptoms for acute myocardial infarction, prolonged PCI (lasting more than 70 minutes) and failed PCI.
Use of Thrombolytics, Anticoagulants and Other Antiplatelet Agents
In the EPIC, EPILOG, CAPTURE, and EPISTENT trials, Abciximab was used concomitantly with heparin and aspirin.
For details of the anticoagulation algorithms used in these clinical trials, see CLINICAL STUDIES: Anticoagulation. Because Abciximab inhibits platelet aggregation, caution should be employed when it is used with other drugs that affect hemostasis, including thrombolytics, oral anticoagulants, non-steroidal anti-inflammatory drugs, dipyridamole, and ticlopidine.
In the EPIC trial, there was limited experience with the administration of Abciximab with low molecular weight dextran. Low molecular weight dextran was usually given for the deployment of a coronary stent, for which oral anticoagulants were also given. In the 11 patients who received low molecular weight dextran with Abciximab, five had major bleeding events and four had minor bleeding events. None of the five placebo patients treated with low molecular weight dextran had a major or minor bleeding event (see CONTRAINDICATIONS). Because of observed synergistic effects on bleeding, Abciximab therapy should be used judiciously in patients who have received systemic thrombolytic therapy. The GUSTO V trial randomized patients with acute myocardial infarction to treatment with combined Abciximab and half-dose Reteplase, or full-dose Reteplase alone (15). In this trial, the incidence of moderate or severe nonintracranial bleeding was increased in those patients receiving Abciximab and half-dose Reteplase versus those receiving Reteplase alone (4.6% versus 2.3%, respectively).
Thrombocytopenia
Thrombocytopenia, including severe thrombocytopenia, has been observed with Abciximab administration (see ADVERSE REACTIONS: Thrombocytopenia). Platelet counts should be monitored prior to, during, and after treatment with Abciximab. Acute decreases in platelet count should be differentiated between true thrombocytopenia and pseudothrombocytopenia (see PRECAUTIONS: Laboratory Tests). If true thrombocytopenia is verified, Abciximab should be immediately discontinued and the condition appropriately monitored and treated.
In clinical trials, patients who developed thrombocytopenia were followed with daily platelet counts until their platelet count returned to normal. Heparin and aspirin were discontinued for platelet counts below 60,000 cells/μL and platelets were transfused for a platelet count below 50,000 cells/μL. Most cases of severe thrombocytopenia (< 50,000 cells/μL) occurred within the first 24 hours of Abciximab administration.
In a registry study of Abciximab readministration, a history of thrombocytopenia associated with prior use of Abciximab was predictive of an increased risk of recurrent thrombocytopenia (see ADVERSE REACTIONS: Thrombocytopenia). Readministration within 30 days was associated with an increased incidence and severity of thrombocytopenia, as was a positive human anti-chimeric antibody (HACA) test at baseline, compared to the rates seen in studies with first administration.
Restoration of Platelet Function- In the event of serious uncontrolled bleeding or the need for emergency surgery, Abciximab should be discontinued. If platelet function does not return to normal, it may be restored, at least in part, with platelet transfusions.
Laboratory Tests
Before infusion of Abciximab, prothrombin time, ACT, APTT, and platelet count should be measured to identify pre-existing hemostatic abnormalities.
Based on an integrated analysis of data from all studies, the following guidelines may be utilized to minimize the risk for bleeding:
- When Abciximab is initiated 18 to 24 hours before PCI, the APTT should be maintained between 60 and 85 seconds during the Abciximab and heparin infusion period.
- If anticoagulation is continued in these patients following PCI, the APTT should be maintained between 55 and 75 seconds.
- The APTT or ACT should be checked prior to arterial sheath removal. The sheath should not be removed unless APTT ≤ 50 seconds or ACT ≤ 175 seconds.
- Platelet counts should be monitored prior to treatment, two to four hours following the bolus dose of Abciximab and at 24 hours or prior to discharge, whichever is first. If a patient experiences an acute platelet decrease (e.g., a platelet decrease to less than 100,000 cells/μL and a decrease of at least 25% from pre-treatment value), additional platelet counts should be determined. Platelet monitoring should continue until platelet counts return to normal.
- To exclude pseudothrombocytopenia, a laboratory artifact due to in vitro anticoagulant interaction, blood samples should be drawn in three separate tubes containing ethylenediaminetetraacetic acid (EDTA), citrate and heparin, respectively. A low platelet count in EDTA but not in heparin and/or citrate is supportive of a diagnosis of pseudothrombocytopenia.
Readministration
Administration of Abciximab may result in the formation of HACA that could potentially cause allergic or hypersensitivity reactions (including anaphylaxis), thrombocytopenia or diminished benefit upon readministration of Abciximab (see WARNINGS: Allergic Reactions; see ADVERSE REACTIONS: Immunogenicity).
Readministration of Abciximab to patients undergoing PCI was assessed in a registry that included 1342 treatments in 1286 patients. Most patients were receiving their second Abciximab exposure; 15% were receiving the third or subsequent exposure. The overall rate of HACA positivity prior to the readministration was 6% and increased to 27% post-readministration. There were no reports of serious allergic reactions or anaphylaxis (see WARNINGS: Allergic Reactions). Thrombocytopenia was observed at higher rates in the readministration study than in the phase 3 studies of first-time administration (see PRECAUTIONS: Thrombocytopenia and Adverse Reactions: Thrombocytopenia), suggesting that readministration may be associated with an increased incidence and severity of thrombocytopenia.
Adverse Reactions
Clinical Trials Experience
Bleeding
Abciximab has the potential to increase the risk of bleeding, particularly in the presence of anticoagulation, e.g., from heparin, other anticoagulants or thrombolytics. Bleeding in the Phase 3 trials was classified as major, minor or insignificant by the criteria of the Thrombolysis in Myocardial Infarction study group (16). Major bleeding events were defined as either an intracranial hemorrhage or a decrease in hemoglobin greater than 5 g/dL. Minor bleeding events included spontaneous gross hematuria, spontaneous hematemesis, observed blood loss with a hemoglobin decrease of more than 3 g/dL, or a decrease in hemoglobin of at least 4 g/dL without an identified bleeding site. Insignificant bleeding events were defined as a decrease in hemoglobin of less than 3 g/dL or a decrease in hemoglobin between 3-4 g/dL without observed bleeding. In patients who received transfusions, the number of units of blood lost was estimated through an adaptation of the method of Landefeld, et al. (17).
In the EPIC trial, in which a non-weight-adjusted, longer-duration heparin dose regimen was used, the most common complication during Abciximab therapy was bleeding during the first 36 hours. The incidences of major bleeding, minor bleeding and transfusion of blood products were significantly increased. Major bleeding occurred in 10.6% of patients in the Abciximab bolus plus infusion arm compared with 3.3% of patients in the placebo arm. Minor bleeding was seen in 16.8% of Abciximab bolus plus infusion patients and 9.2% of placebo patients (7). Approximately 70% of Abciximab-treated patients with major bleeding had bleeding at the arterial access site in the groin. Abciximab-treated patients also had a higher incidence of major bleeding events from gastrointestinal, genitourinary, retroperitoneal, and other sites.
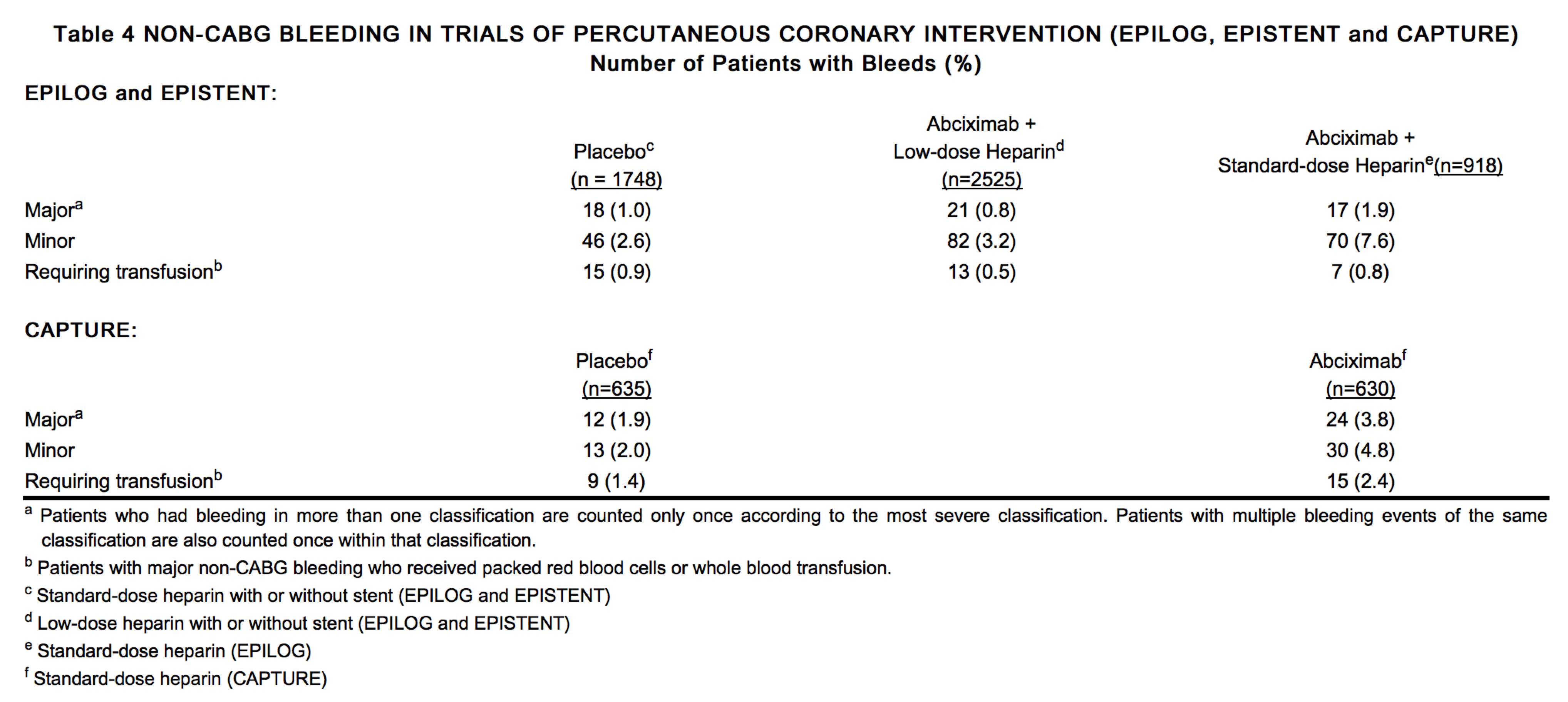
Bleeding rates were reduced in the CAPTURE trial, and further reduced in the EPILOG and EPISTENT trials by use of modified dosing regimens and specific patient management techniques. In EPILOG and EPISTENT, using the heparin and Abciximab dosing, sheath removal and arterial access site guidelines described under PRECAUTIONS, the incidence of major bleeding in patients treated with Abciximab and low-dose, weight-adjusted heparin was not significantly different from that in patients receiving placebo.
Subgroup analyses in the EPIC and CAPTURE trials showed that non-CABG major bleeding was more common in Abciximab patients weighing ≤ 75 kg. In the EPILOG and EPISTENT trials, which used weight-adjusted heparin dosing, the non-CABG major bleeding rates for Abciximab-treated patients did not differ substantially by weight subgroup.
Although data are limited, Abciximab treatment was not associated with excess major bleeding in patients who underwent CABG surgery. (The range among all treatment arms was 3-5% in EPIC, and 1-2% in the CAPTURE, EPILOG, and EPISTENT trials.) Some patients with prolonged bleeding times received platelet transfusions to correct the bleeding time prior to surgery. (see PRECAUTIONS: Restoration of Platelet Function.)
The rates of major bleeding, minor bleeding and bleeding events requiring transfusions in the CAPTURE, EPILOG, and EPISTENT trials are shown in Table 4. The rates of insignificant bleeding events are not included in Table 4.
Cases of fatal bleeding have been reported rarely during post-marketing use of Abciximab (see WARNINGS: Bleeding Events).
Pulmonary alveolar hemorrhage has been rarely reported during use of Abciximab. This can present with any or all of the following in close association with ReoPro administration: hypoxemia, alveolar infiltrates on chest x-ray, hemoptysis, or an unexplained drop in hemoglobin.
Intracranial Hemorrhage and Stroke
The total incidence of intracranial hemorrhage and non-hemorrhagic stroke across all four trials was not significantly different, 9/3023 for placebo patients and 15/4680 for Abciximab-treated patients. The incidence of intracranial hemorrhage was 3/3023 for placebo patients and 7/4680 for Abciximab patients.
Thrombocytopenia
In the clinical trials, patients treated with Abciximab were more likely than patients treated with placebo to experience decreases in platelet counts.
Among patients in the EPILOG and EPISTENT trials who were treated with Abciximab plus low-dose heparin, the proportion of patients with any thrombocytopenia (platelets less than 100,000 cells/μL) ranged from 2.5 to 3.0%. The incidence of severe thrombocytopenia (platelets less than 50,000 cells/μL) ranged from 0.4 to 1.0% and platelet transfusions were required in 0.9 to 1.1%, respectively. Modestly lower rates were observed among patients treated with placebo plus standard-dose heparin. Overall higher rates were observed among patients in the EPIC and CAPTURE trials treated with Abciximab plus longer duration heparin: 2.6 to 5.2% were found to have any thrombocytopenia, 0.9 to 1.7% had severe thrombocytopenia, and 2.1 to 5.5% required platelet transfusion, respectively.
In a readministration registry study of patients receiving a second or subsequent exposure to Abciximab (see PRECAUTIONS: Readministration) the incidence of any degree of thrombocytopenia was 5%, with an incidence of profound thrombocytopenia of 2% (<20,000 cell/μL). Factors associated with an increased risk of thrombocytopenia were a history of thrombocytopenia on previous Abciximab exposure, readministration within 30 days, and a positive HACA assay prior to the readministration.
Among 14 patients who had thrombocytopenia associated with a prior exposure to Abciximab, 7 (50%) had recurrent thrombocytopenia. In 130 patients with a readministration interval of 30 days or less, 25 (19%) developed thrombocytopenia. Severe thrombocytopenia occurred in 19 of these patients. Among the 71 patients who had a positive HACA assay at baseline, 11 (15%) developed thrombocytopenia, 7 of which were severe.
Allergic Reactions
There have been rare reports of allergic reactions, some of which were anaphylaxis (see WARNINGS: Allergic Reactions).
Other Adverse Reactions
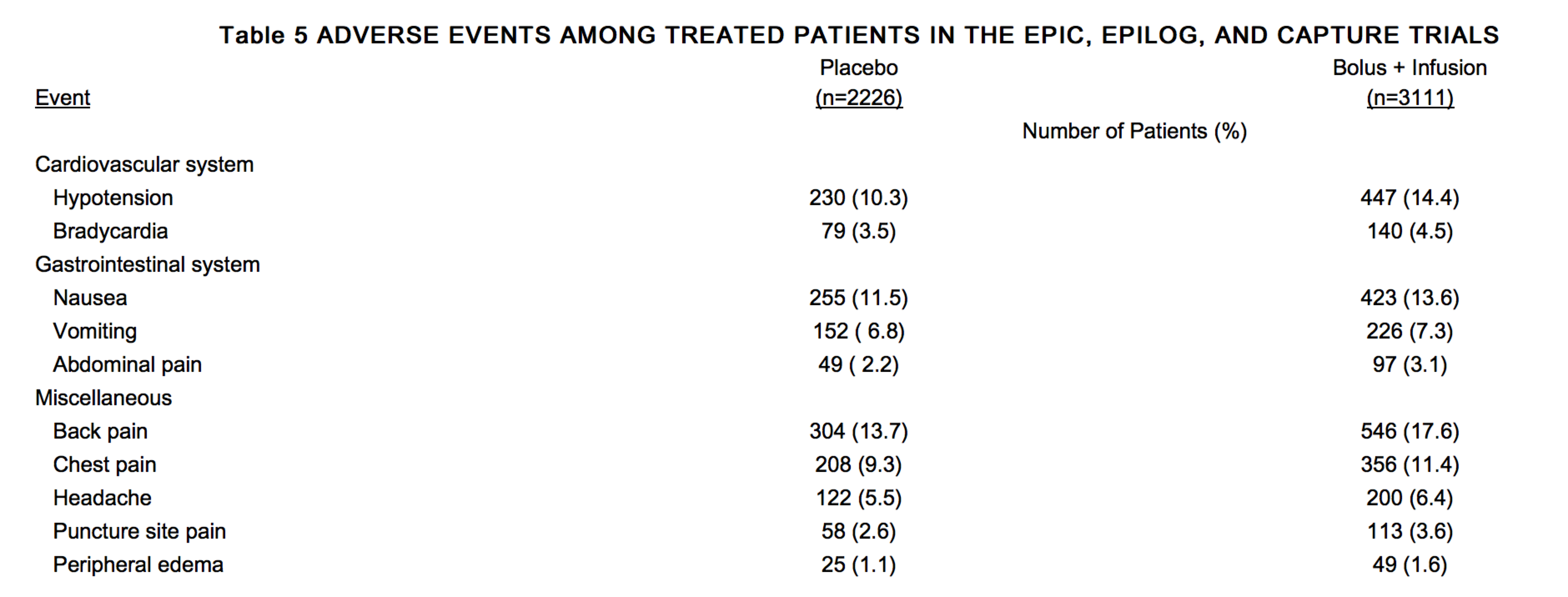
Table 5 shows adverse events other than bleeding and thrombocytopenia from the combined EPIC, EPILOG and CAPTURE trials which occurred in patients in the bolus plus infusion arm at an incidence of more than 0.5% higher than in those treated with placebo.
The following additional adverse events from the EPIC, EPILOG and CAPTURE trials were reported by investigators for patients treated with a bolus plus infusion of Abciximab at incidences which were less than 0.5% higher than for patients in the placebo arm.
Cardiovascular System: ventricular tachycardia (1.4%), pseudoaneurysm (0.8%), palpitation (0.5%), arteriovenous fistula (0.4%), incomplete AV block (0.3%), nodal arrhythmia (0.2%), complete AV block (0.1%), embolism (limb)(0.1%); thrombophlebitis (0.1%);
Gastrointestinal System: dyspepsia (2.1%), diarrhea (1.1%), ileus (0.1%), gastroesophogeal reflux (0.1%);
Hemic and Lymphatic System: anemia (1.3%), leukocytosis (0.5%), petechiae (0.2%);
Nervous System: dizziness (2.9%), anxiety (1.7%), abnormal thinking (1.3%), agitation (0.7%), hypesthesia (0.6%), confusion (0.5%) muscle contractions (0.4%), coma (0.2%), hypertonia (0.2%), diplopia (0.1%);
Respiratory System: pneumonia (0.4%), rales (0.4%), pleural effusion (0.3%), bronchitis (0.3%) bronchospasm (0.3%), pleurisy (0.2%), pulmonary embolism (0.2%), rhonchi (0.1%);
Musculoskeletal System: myalgia (0.2%);
Urogenital System: urinary retention (0.7%), dysuria (0.4%), abnormal renal function (0.4%), frequent micturition (0.1%), cystalgia (0.1%), urinary incontinence (0.1%), prostatitis (0.1%);
Miscellaneous: pain (5.4%), sweating increased (1.0%), asthenia (0.7%), incisional pain (0.6%), pruritus (0.5%), abnormal vision (0.3%), edema (0.3%), wound (0.2%), abscess (0.2%), cellulitis (0.2%), peripheral coldness (0.2%), injection site pain (0.1%), dry mouth (0.1%), pallor (0.1%), diabetes mellitus (0.1%), hyperkalemia (0.1%), enlarged abdomen (0.1%), bullous eruption (0.1%), inflammation (0.1%), drug toxicity (0.1%).
Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. In the EPIC, EPILOG, and CAPTURE trials, positive HACA responses occurred in approximately 5.8% of these patients receiving a first exposure to Abciximab. No increase in hypersensitivity or allergic reactions was observed with Abciximab treatment (see WARNINGS: Allergic Reactions).
In a study of readministration of Abciximab to patients (see PRECAUTIONS: Readministration) the overall rate of HACA positivity prior to the readministration was 6% and increased post-readministration to 27%. Among the 36 subjects receiving a fourth or greater Abciximab exposure, HACA positive assays were observed post-readministration in 16 subjects (44%).
There were no reports of serious allergic reactions or anaphylaxis (see WARNINGS: Allergic Reactions). HACA positive status was associated with an increased risk of thrombocytopenia (see PRECAUTIONS: Thrombocytopenia).
The data reflect the percentage of patients whose test results were considered positive for antibodies to Abciximab using an ELISA assay, and are highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody positivity in an assay may be influenced by several factors including sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Abciximab with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
There is limited information regarding Sandbox Abciximab Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Sandbox Abciximab Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Sandbox Abciximab in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sandbox Abciximab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Sandbox Abciximab during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Sandbox Abciximab in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Sandbox Abciximab in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Sandbox Abciximab in geriatric settings.
Gender
There is no FDA guidance on the use of Sandbox Abciximab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sandbox Abciximab with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Sandbox Abciximab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Sandbox Abciximab in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sandbox Abciximab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sandbox Abciximab in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Sandbox Abciximab Administration in the drug label.
Monitoring
There is limited information regarding Sandbox Abciximab Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Sandbox Abciximab and IV administrations.
Overdosage
There is limited information regarding Sandbox Abciximab overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Sandbox Abciximab Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Sandbox Abciximab Mechanism of Action in the drug label.
Structure
There is limited information regarding Sandbox Abciximab Structure in the drug label.
Pharmacodynamics
There is limited information regarding Sandbox Abciximab Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Sandbox Abciximab Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Sandbox Abciximab Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Sandbox Abciximab Clinical Studies in the drug label.
How Supplied
There is limited information regarding Sandbox Abciximab How Supplied in the drug label.
Storage
There is limited information regarding Sandbox Abciximab Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Sandbox Abciximab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Sandbox Abciximab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Sandbox Abciximab Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Sandbox Abciximab interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Sandbox Abciximab Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Sandbox Abciximab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Giri S, Mitchel J, Azar RR, Kiernan FJ, Fram DB, McKay RG et al. (2002) Results of primary percutaneous transluminal coronary angioplasty plus abciximab with or without stenting for acute myocardial infarction complicated by cardiogenic shock. Am J Cardiol 89 (2):126-31. PMID: 11792329
- ↑ Duda SH, Tepe G, Luz O, Ouriel K, Dietz K, Hahn U et al. (2001) Peripheral artery occlusion: treatment with abciximab plus urokinase versus with urokinase alone--a randomized pilot trial (the PROMPT Study). Platelet Receptor Antibodies in Order to Manage Peripheral Artery Thrombosis. Radiology 221 (3):689-96. DOI:10.1148/radiol.2213010400 PMID: 11719664
- ↑ Abciximab in Ischemic Stroke Investigators (2000) Abciximab in acute ischemic stroke. A randomized, double-blind, placebo-controlled, dose-escalation study. Stroke 31 (3):601-9. PMID: 10700492
- ↑ (2000) Trial of abciximab with and without low-dose reteplase for acute myocardial infarction. Strategies for Patency Enhancement in the Emergency Department (SPEED) Group. Circulation 101 (24):2788-94. PMID: 10859283
- ↑ Williams RV, Wilke VM, Tani LY, Minich LL (2002) Does Abciximab enhance regression of coronary aneurysms resulting from Kawasaki disease? Pediatrics 109 (1):E4. PMID: 11773572