Tipranavir warnings and precautions
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Warnings And Precautions
Please refer to the ritonavir prescribing information for additional information on precautionary measures.
Hepatic Impairment and Toxicity
Clinical hepatitis and hepatic decompensation, including some fatalities, were reported with APTIVUS co-administered with 200 mg of ritonavir. These have generally occurred in patients with advanced HIV-1 disease taking multiple concomitant medications. A causal relationship to APTIVUS/ritonavir could not be established. Physicians and patients should be vigilant for the appearance of signs or symptoms of hepatitis, such as fatigue, malaise, anorexia, nausea, jaundice, bilirubinuria, acholic stools, liver tenderness or hepatomegaly. Patients with signs or symptoms of clinical hepatitis should discontinue APTIVUS/ritonavir treatment and seek medical evaluation.
All patients should be followed closely with clinical and laboratory monitoring, especially those with chronic hepatitis B or C co-infection, as these patients have an increased risk of hepatotoxicity. Liver function tests should be performed prior to initiating therapy with APTIVUS/ritonavir, and frequently throughout the duration of treatment.
If asymptomatic elevations in AST or ALT greater than 10 times the upper limit of normal occur, APTIVUS/ritonavir therapy should be discontinued. If asymptomatic elevations in AST or ALT between 5 – 10 times the upper limit of normal and increases in total bilirubin greater than 2.5 times the upper limit of normal occur, APTIVUS/ritonavir therapy should be discontinued.
Treatment-experienced patients with chronic hepatitis B or hepatitis C co-infection or elevated transaminases are at approximately 2-fold risk for developing Grade 3 or 4 transaminase elevations or hepatic decompensation. In two large, randomized, open-label, controlled clinical trials with an active comparator (1182.12 and 1182.48) of treatment-experienced patients, Grade 3 and 4 increases in hepatic transaminases were observed in 10.3% (10.9/100 PEY) receiving APTIVUS/ritonavir through week 48. In a study of treatment-naïve patients, 20.3% (21/100 PEY) experienced Grade 3 or 4 hepatic transaminase elevations while receiving APTIVUS/ritonavir 500 mg/200 mg through week 48.
Tipranavir is principally metabolized by the liver. Caution should be exercised when administering APTIVUS/ritonavir to patients with mild hepatic impairment (Child-Pugh Class A) because tipranavir concentrations may be increased [see Clinical Pharmacology (12.3)].
Intracranial Hemorrhage
APTIVUS, co-administered with 200 mg of ritonavir, has been associated with reports of both fatal and non-fatal intracranial hemorrhage (ICH). Many of these patients had other medical conditions or were receiving concomitant medications that may have caused or contributed to these events. No pattern of abnormal coagulation parameters has been observed in patients in general, or preceding the development of ICH. Therefore, routine measurement of coagulation parameters is not currently indicated in the management of patients on APTIVUS.
Drug Interactions
See Table 1 for a listing of contraindicated drugs with APTIVUS/ritonavir due to potentially life-threatening adverse events, significant drug interactions, or due to loss of virologic activity [see Contraindications (4.2)]. See Table 4 for a listing of established and other potentially significant drug interactions with APTIVUS/ritonavir.
Effects on Platelet Aggregation and Coagulation
APTIVUS/ritonavir should be used with caution in patients who may be at risk of increased bleeding from trauma, surgery or other medical conditions, or who are receiving medications known to increase the risk of bleeding such as antiplatelet agents and anticoagulants, or who are taking supplemental high doses of vitamin E.
In rats, tipranavir treatment alone induced dose-dependent changes in coagulation parameters, bleeding events and death. Co-administration with vitamin E significantly increased these effects [see Nonclinical Toxicology (13.2)]. However, analyses of stored plasma from adult patients treated with APTIVUS capsules and pediatric patients treated with APTIVUS oral solution (which contains a vitamin E derivative) showed no effect of APTIVUS/ritonavir on vitamin K-dependent coagulation factors (Factor II and Factor VII), Factor V, or on prothrombin or activated partial thromboplastin times.
In in vitro experiments, tipranavir was observed to inhibit human platelet aggregation at levels consistent with exposures observed in patients receiving APTIVUS/ritonavir.
Vitamin E Intake
Patients taking APTIVUS oral solution should be advised not to take supplemental vitamin E greater than a standard multivitamin as APTIVUS oral solution contains 116 IU/mL of vitamin E which is higher than the Reference Daily Intake (adults 30 IU, pediatrics approximately 10 IU).
Rash
Rash, including urticarial rash, maculopapular rash, and possible photosensitivity, has been reported in subjects receiving APTIVUS/ritonavir. In some cases rash was accompanied by joint pain or stiffness, throat tightness, or generalized pruritus. In controlled adult clinical trials, rash (all grades, all causality) was observed in 10% of females and in 8% of males receiving APTIVUS/ritonavir through 48 weeks of treatment. The median time to onset of rash was 53 days and the median duration of rash was 22 days. The discontinuation rate for rash in clinical trials was 0.5%. In an uncontrolled compassionate use program (n=3920), cases of rash, some of which were severe, accompanied by myalgia, fever, erythema, desquamation, and mucosal erosions were reported. In the pediatric clinical trial, the frequency of rash (all grades, all causality) through 48 weeks of treatment was 21%. Overall, most of the pediatric patients had mild rash and 5 (5%) had moderate rash. Overall 3% of pediatric patients interrupted APTIVUS treatment due to rash and the discontinuation rate for rash in pediatric patients was 0.9%. Discontinue and initiate appropriate treatment if severe skin rash develops.
Sulfa Allergy
APTIVUS should be used with caution in patients with a known sulfonamide allergy. Tipranavir contains a sulfonamide moiety. The potential for cross-sensitivity between drugs in the sulfonamide class and APTIVUS is unknown.
Diabetes Mellitus/Hyperglycemia
New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus and hyperglycemia have been reported during post-marketing surveillance in HIV-1 infected patients receiving protease inhibitor therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those patients who discontinued protease inhibitor therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and a causal relationship between protease inhibitor therapy and these events has not been established.
Immune Reconstitution Syndromev
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including APTIVUS. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium aviuminfection, cytomegalovirus, Pneumocystis jiroveci pneumonia, tuberculosis, or reactivation of herpes simplex and herpes zoster), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution, however, the time to onset is more variable, and can occur many months after initiation of treatment.
Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
Elevated Lipids
Treatment with APTIVUS co-administered with 200 mg of ritonavir has resulted in large increases in the concentration of total cholesterol and triglycerides [see Adverse Reactions (6)]. Triglyceride and cholesterol testing should be performed prior to initiating APTIVUS/ritonavir therapy and at periodic intervals during therapy. Lipid disorders should be managed as clinically appropriate; taking into account any potential drug-drug interactions [see Drug Interactions (7.2)].
Patients with Hemophilia
There have been reports of increased bleeding, including spontaneous skin hematomas and hemarthrosis in patients with hemophilia type A and B treated with protease inhibitors. In some patients additional Factor VIII was given. In more than half of the reported cases, treatment with protease inhibitors was continued or reintroduced if treatment had been discontinued. A causal relationship between protease inhibitors and these events has not been established.
5.13 Resistance/Cross Resistance
Because the potential for HIV-1 cross-resistance among protease inhibitors has not been fully explored in APTIVUS/ritonavir treated patients, it is unknown what effect therapy with APTIVUS will have on the activity of subsequently administered protease inhibitors.
6 ADVERSE REACTIONS===
The following adverse reactions are described, in greater detail, in other sections:
Hepatic Impairment and Toxicity [see Warnings and Precautions (5.1)] Intracranial Hemorrhage [see Warnings and Precautions (5.2)] Rash [see Warnings and Precautions (5.6)]
Due to the need for co-administration of APTIVUS with ritonavir, please refer to ritonavir prescribing information for ritonavir-associated adverse reactions.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Clinical Trials in Adults
APTIVUS, co-administered with ritonavir, has been studied in a total of 6308 HIV-1 positive adults as combination therapy in clinical studies. Of these, 1299 treatment-experienced patients received the dose of 500/200 mg BID. Nine hundred nine (909) adults, including 541 in the 1182.12 and 1182.48 controlled clinical trials, have been treated for at least 48 weeks [see Clinical Studies (14)].
In 1182.12 and 1182.48 in the APTIVUS/ritonavir arm, the most frequent adverse reactions were diarrhea, nausea, pyrexia, vomiting, fatigue, headache, and abdominal pain. The 48-Week Kaplan-Meier rates of adverse reactions leading to discontinuation were 13.3% for APTIVUS/ritonavir-treated patients and 10.8% for the comparator arm patients.
Adverse reactions reported in the controlled clinical trials 1182.12 and 1182.48, based on treatment-emergent clinical adverse reactions of moderate tNNRXXo severe intensity (Grades 2 - 4) in at least 2% of treatment-experienced subjects in either treatment group are summarized in Table 2 below.[1]
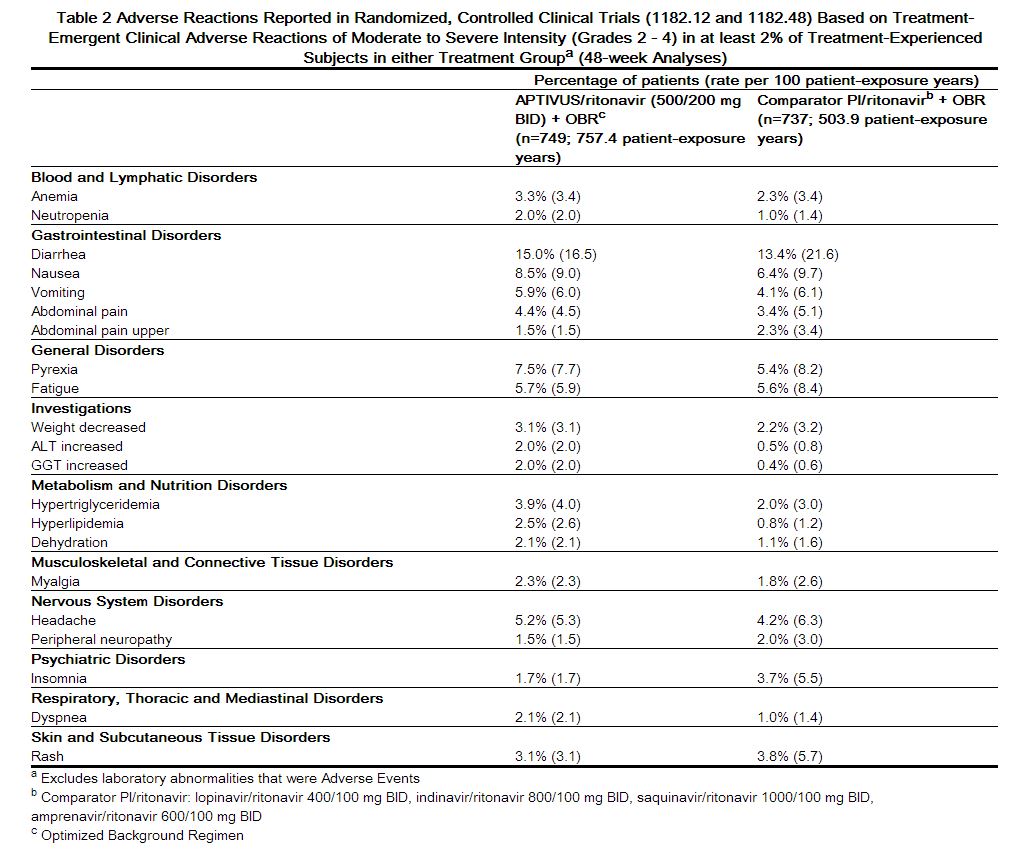 |
References
Adapted from the FDA Package Insert.