Tipranavir clinical pharmacology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Clinical Pharmacology
Mechanism of Action
Tipranavir is an antiviral drug.
Pharmacodynamics
ECG Evaluation The effect of APTIVUS/ritonavir on the QTcF interval was measured in a study in which 81 healthy subjects received the following treatments twice daily for 2.5 days: APTIVUS/ritonavir (500/200 mg), APTIVUS/ritonavir at a supra-therapeutic dose (750/200 mg), and placebo/ritonavir (-/200 mg). After baseline and placebo adjustment, the maximum mean QTcF change was 3.2 ms (1-sided 95% Upper CI: 5.6 ms) for the 500/200 mg dose and 8.3 ms (1-sided 95% Upper CI: 10.9 ms) for the supra-therapeutic 750/200 mg dose.
Antiviral Activity in vivo The median Inhibitory Quotient (IQ) determined from 264 treatment-experienced adult patients was about 80 (inter-quartile range: 31-226), from the controlled clinical trials 1182.12 and 1182.48. The IQ is defined as the tipranavir trough concentration divided by the viral EC50 value, corrected for protein binding. There was a relationship between the proportion of patients with a ≥1 log10 reduction of viral load from baseline at week 48 and their IQ value. Among the 198 patients receiving APTIVUS/ritonavir with no new enfuvirtide use (e.g., new enfuvirtide, defined as initiation of enfuvirtide for the first time), the response rate was 23% in those with an IQ value <80 and 59% in those with an IQ value ≥80. Among the 66 patients receiving APTIVUS/ritonavir with new enfuvirtide, the response rates in patients with an IQ value <80 versus those with an IQ value ≥80 were 55% and 71%, respectively. These IQ groups are derived from a select population and are not meant to represent clinical breakpoints.
Pharmacokinetics
In order to achieve effective tipranavir plasma concentrations and a twice-daily dosing regimen, co-administration of APTIVUS with ritonavir is essential. Ritonavir inhibits hepatic cytochrome P450 3A (CYP 3A), the intestinal P-gp efflux pump and possibly intestinal CYP 3A. In a dose-ranging evaluation in 113 HIV-1 negative male and female volunteers, there was a 29-fold increase in the geometric mean morning steady-state trough plasma concentrations of tipranavir following APTIVUS co-administered with low-dose ritonavir (500/200 mg twice daily) compared to APTIVUS 500 mg twice daily without ritonavir. In adults the mean systemic ritonavir concentration when 200 mg of ritonavir was given with 500 mg of APTIVUS was similar to the concentrations observed when 100 mg was given with the other protease inhibitors.
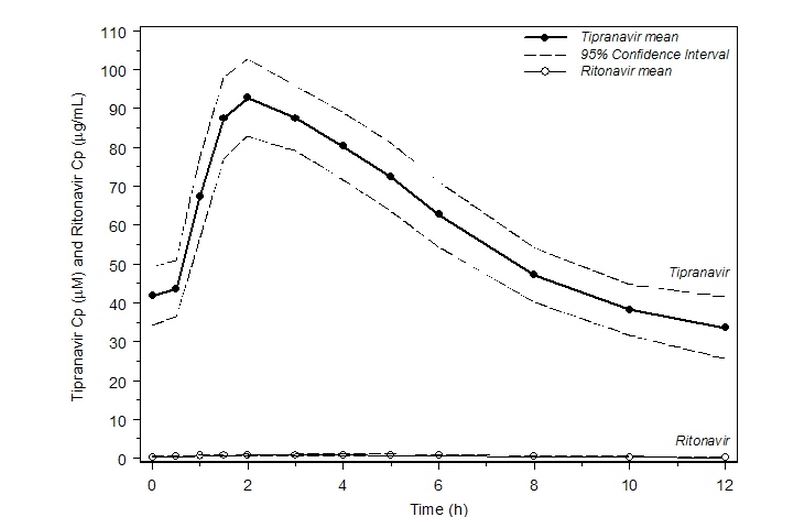
Figure 1 displays mean plasma concentrations of tipranavir and ritonavir at steady state for 30 HIV-1 infected adult patients dosed with 500/200 mg tipranavir/ritonavir for 14 days.
Figure 1 Mean Steady State Tipranavir Plasma Concentrations (95% CI) with Ritonavir Co-administration (tipranavir/ritonavir 500/200 mg BID)[1]
|
Absorption and Bioavailability
Absorption of tipranavir in humans is limited, although no absolute quantification of absorption is available. Tipranavir is a P-gp substrate, a weak P-gp inhibitor, and appears to be a potent P-gp inducer as well. In vivo data suggest that tipranavir/ritonavir, at the dose of 500/200 mg, is a P-gp inhibitor after the first dose and induction of P-gp occurs over time. Tipranavir trough concentrations at steady-state are about 70% lower than those on Day 1, presumably due to intestinal P-gp induction. Steady state is attained in most subjects after 7-10 days of dosing.
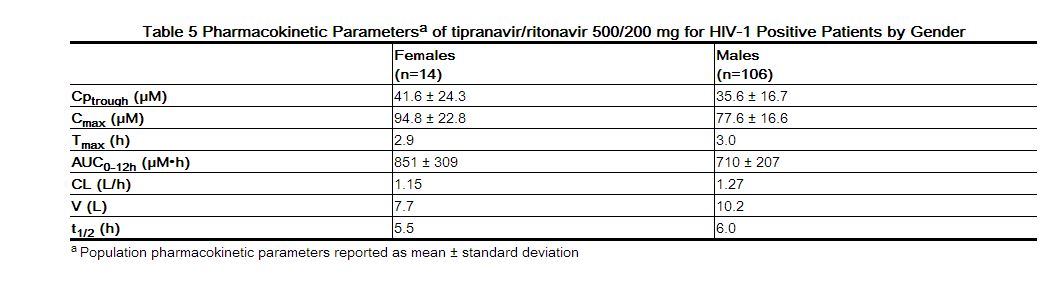
Dosing APTIVUS 500 mg with 200 mg ritonavir capsules twice daily for greater than 2 weeks and without meal restriction produced the pharmacokinetic parameters for male and female HIV-1 positive patients presented in Table 5.
|
Effects of Food on Oral Absorption
For APTIVUS capsules or oral solution co-administered with ritonavir capsules at steady-state, no clinically significant changes in tipranavir Cmax, Cp12h, and AUC were observed under fed conditions (500-682 Kcal, 23-25% calories from fat) compared to fasted conditions [see Dosage and Administration (2)]. The effect of food on tipranavir exposure when APTIVUS capsules or oral solution is co-administered with ritonavir tablets has not been evaluated [see Dosage and Administration (2)]. For information on the effect of food on the bioavailability of ritonavir tablets, please refer to the ritonavir tablet prescribing information.
Distribution
Tipranavir is extensively bound to plasma proteins (>99.9%). It binds to both human serum albumin and α-1-acid glycoprotein. The mean fraction of tipranavir (dosed without ritonavir) unbound in plasma was similar in clinical samples from healthy volunteers and HIV-1 positive patients. Total plasma tipranavir concentrations for these samples ranged from 9 to 82 μM. The unbound fraction of tipranavir appeared to be independent of total drug concentration over this concentration range.
No studies have been conducted to determine the distribution of tipranavir into human cerebrospinal fluid or semen.
Metabolism
In vitro metabolism studies with human liver microsomes indicated that CYP 3A4 is the predominant CYP enzyme involved in tipranavir metabolism.
The oral clearance of tipranavir decreased after the addition of ritonavir, which may represent diminished first-pass clearance of the drug at the gastrointestinal tract as well as the liver.
The metabolism of tipranavir in the presence of 200 mg ritonavir is minimal. Administration of 14C-tipranavir to subjects that received APTIVUS/ritonavir 500/200 mg dosed to steady-state demonstrated that unchanged tipranavir accounted for 98.4% or greater of the total plasma radioactivity circulating at 3, 8, or 12 hours after dosing. Only a few metabolites were found in plasma, and all were at trace levels (0.2% or less of the plasma radioactivity). In feces, unchanged tipranavir represented the majority of fecal radioactivity (79.9% of fecal radioactivity). The most abundant fecal metabolite, at 4.9% of fecal radioactivity (3.2% of dose), was a hydroxyl metabolite of tipranavir. In urine, unchanged tipranavir was found in trace amounts (0.5% of urine radioactivity). The most abundant urinary metabolite, at 11.0% of urine radioactivity (0.5% of dose) was a glucuronide conjugate of tipranavir.
Elimination
Administration of 14C-tipranavir to subjects (n=8) that received APTIVUS/ritonavir 500/200 mg dosed to steady-state demonstrated that most radioactivity (median 82.3%) was excreted in feces, while only a median of 4.4% of the radioactive dose administered was recovered in urine. In addition, most radioactivity (56%) was excreted between 24 and 96 hours after dosing. The effective mean elimination half-life of tipranavir/ritonavir in healthy volunteers (n=67) and HIV-1 infected adult patients (n=120) was approximately 4.8 and 6.0 hours, respectively, at steady state following a dose of 500/200 mg twice daily with a light meal.
Special Populations
Renal Impairment APTIVUS pharmacokinetics have not been studied in patients with renal dysfunction. However, since the renal clearance of tipranavir is negligible, a decrease in total body clearance is not expected in patients with renal insufficiency.
Hepatic Impairment In a study comparing 9 HIV-1 negative patients with mild (Child-Pugh Class A) hepatic impairment to 9 HIV-1 negative controls, the single and multiple dose plasma concentrations of tipranavir and ritonavir were increased in patients with hepatic impairment, but were within the range observed in clinical trials. No dosing adjustment is required in patients with mild hepatic impairment.
The influence of moderate hepatic impairment (Child-Pugh Class B) or severe hepatic impairment (Child-Pugh Class C) on the multiple-dose pharmacokinetics of tipranavir administered with ritonavir has not been evaluated.
Gender Evaluation of steady-state plasma tipranavir trough concentrations at 10-14 h after dosing from the controlled clinical trials 1182.12 and 1182.48 demonstrated that females generally had higher tipranavir concentrations than males. After 4 weeks of APTIVUS/ritonavir 500/200 mg BID, the median plasma trough concentration of tipranavir was 43.9 μM for females and 31.1 μM for males. The difference in concentrations does not warrant a dose adjustment.
Race Evaluation of steady-state plasma tipranavir trough concentrations at 10-14 h after dosing from the controlled clinical trials 1182.12 and 1182.48 demonstrated that white males generally had more variability in tipranavir concentrations than black males, but the median concentration and the range making up the majority of the data are comparable between the races.
Geriatric Patients Evaluation of steady-state plasma tipranavir trough concentrations at 10-14 h after dosing from the controlled clinical trials 1182.12 and 1182.48 demonstrated that there was no change in median trough tipranavir concentrations as age increased for either gender through 65 years of age. There were an insufficient number of women greater than age 65 years in the two trials to evaluate the elderly.
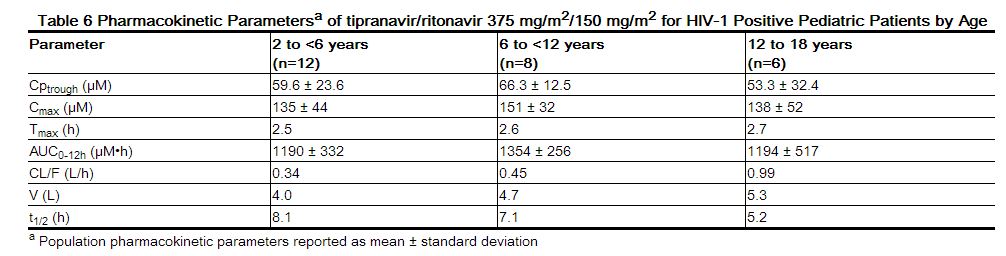
Pediatric Patients Among pediatric patients in clinical trial 1182.14, steady-state plasma tipranavir trough concentrations were obtained 10 to 14 hours following study drug administration. Pharmacokinetic parameters by age group are presented in Table 6.
|
References
Adapted from the FDA Package Insert.