Keratoacanthoma
|
WikiDoc Resources for Keratoacanthoma |
|
Articles |
|---|
|
Most recent articles on Keratoacanthoma Most cited articles on Keratoacanthoma |
|
Media |
|
Powerpoint slides on Keratoacanthoma |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Keratoacanthoma at Clinical Trials.gov Trial results on Keratoacanthoma Clinical Trials on Keratoacanthoma at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Keratoacanthoma NICE Guidance on Keratoacanthoma
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Keratoacanthoma Discussion groups on Keratoacanthoma Patient Handouts on Keratoacanthoma Directions to Hospitals Treating Keratoacanthoma Risk calculators and risk factors for Keratoacanthoma
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Keratoacanthoma |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2] Homa Najafi, M.D.[3]
Overview
Keratoacanthoma (KA) is a relatively common, benign, epithelial tumor that was previously considered to be a variant of squamous cell carcinoma (SCC). The etiology is unknown. No human papillomavirus-DNA sequences were detected in lesions by polymerase chain reaction. It is a disease of the elderly (mean age, 64 years) with an annual incidence rate of 104 per 100,000. It is not associated with internal malignancy. There may be a seasonal presentation of keratoacanthoma that suggests that ultraviolet radiation has an acute effect on the development of KA. KAs may develop in sites of previous trauma. Most cases are the “crateriform” type, which grow rapidly then undergo spontaneous regression. Less than 2% belong to the rare destructive variants with no regression and persistent invasive growth. These are referred to as keratoacanthoma marginatum centrifugum and mutilating keratoacanthomas and can lead to severe defects.
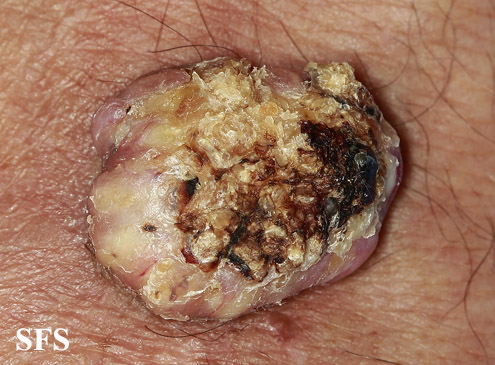
KA begins as a smooth, dome-shaped, red papule that resembles molluscum contagiosum. In a few weeks the tumor may rapidly expand to 1 or 2cm and develop a central keratin-filled crater that is frequently filled with crust. The growth retains its smooth surface, unlike a squamous cell carcinoma. Untreated, growth stops in approximately 6 weeks, and the tumor remains unchanged for an indefinite period. In the majority of cases it then regresses slowly over 2 to 12 months and frequently heals with scarring. The limbs, particularly the sun-exposed hands and arms, are the most common site; the trunk is the second most common site, but KA may occur on any skin surface, including the anal area. On occasion, multiple KAs appear, or a single lesion extends over several centimeters. These variants resist treatment and are unlikely to undergo spontaneous emission.
According to a review of literature by Robert A. Schwartz, KA was once considered a benign neoplasm that resembled a highly malignant one (pseudomalignancy), but it is now viewed in an opposite light as a cancer that resembles a benign neoplasm (pseudobenignity). KA is an abortive malignancy that rarely progresses into an invasive SCC. The KA may serve as a marker for the important autosomal dominant familial cancer syndrome, the Muir-Torre syndrome, as a result of a defective DNA mismatch repair gene.[4]
Diagnosis
Physical Examination
Skin
Face
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
Extremities
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.<ref name="Dermatology
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
Ear
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
Scalp
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
-
Keratoacanthoma. Adapted from Dermatology Atlas.[1]
Keratoacanthoma Pendulum
-
keratoacanthomapendulum. Adapted from Dermatology Atlas.[1]
-
keratoacanthomapendulum. Adapted from Dermatology Atlas.[1]
-
keratoacanthomapendulum. Adapted from Dermatology Atlas.[1]
-
keratoacanthomapendulum. Adapted from Dermatology Atlas.[1]
-
keratoacanthomapendulum. Adapted from Dermatology Atlas.[1]
Keratoacanthoma-Linear Epidermal Nevus
-
keratoacanthoma linearepidermalnevus. Adapted from Dermatology Atlas.[1]
-
keratoacanthoma linearepidermalnevus. Adapted from Dermatology Atlas.[1]
-
keratoacanthoma linearepidermalnevus. Adapted from Dermatology Atlas.[1]
-
keratoacanthoma linearepidermalnevus. Adapted from Dermatology Atlas.[1]
Differentiating keratoacanthoma from other Diseases
| Diseases | Skin examination | Diagnosis | Additional findings | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Color | Texture | Size | Distribution | Dermoscopic Findings | Histopathology | ||||
| Keratoacanthoma[2] |
|
|
|
|
|
| ||||
| Merkel cell carcinoma[3] |
|
|
|
|
|
|
|
|||
| Dermatofibroma[4][5] |
|
|
|
|
|
|||||
| dermatofibrosarcoma protuberans[6][7] |
|
|
|
|
|
|||||
| Kaposi sarcoma[8][9] |
|
|
|
|
| |||||
| Cutaneous squamous cell carcinoma[10] | SCC in situ (Bowen's disease) |
|
|
|
|
|
|
| ||
| Invasive squamous cell carcinoma |
|
|
|
|
|
|||||
| Basal cell carcinoma[11] | Nodular basal cell carcinoma |
|
|
|
|
|
| |||
| Superficial basal cell carcinoma |
|
|
|
| ||||||
| Sclerosing basal cell carcinoma (morpheaform)[12] |
|
|
|
|
|
| ||||
| Prurigo nodules[13][14] |
|
|
|
|
|
|
| |||
| Melanoma[15] | Melanoma in situ (Lentigo Maligna)[16] |
|
|
|
|
|
| |||
| Lentigo maligna melanoma[17] |
|
|
|
|
|
|
| |||
| Superficial spreading melanoma[18] |
|
|
|
|
|
|
||||
| Nodular melanoma[19][20] |
|
|
|
|
||||||
| Acral lentiginous melanoma[21] |
|
|
|
|
|
| ||||
| Amelanotic melanoma[22] |
|
|
|
|
||||||
| Common nevus[23][24] |
|
|
|
|
| |||||
| Blue nevus[25] |
|
|
|
|
|
| ||||
| Spitz nevus[26][27] | Nonpigmented Spitz nevus |
|
|
|
|
| ||||
| Reed-like Spitz[28] |
|
|
|
|
|
|
| |||
| Solar lentigo[29] |
|
|
|
|
|
|
|
| ||
| Sebaceous hyperplasia[30] |
|
|
|
| ||||||
| Lichen planus-like keratosis[31] |
|
|
|
|
|
| ||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 1.28 1.29 1.30 1.31 1.32 1.33 1.34 1.35 1.36 1.37 1.38 1.39 1.40 1.41 1.42 1.43 1.44 1.45 1.46 1.47 1.48 1.49 1.50 1.51 1.52 1.53 1.54 1.55 "Dermatology Atlas".
- ↑ Kwiek B, Schwartz RA (2016). "Keratoacanthoma (KA): An update and review". J Am Acad Dermatol. 74 (6): 1220–33. doi:10.1016/j.jaad.2015.11.033. PMID 26853179.
- ↑ Albores-Saavedra J, Batich K, Chable-Montero F, Sagy N, Schwartz AM, Henson DE (2010). "Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study". J Cutan Pathol. 37 (1): 20–7. doi:10.1111/j.1600-0560.2009.01370.x. PMID 19638070.
- ↑ Lee, MiWoo; Lee, WooJin; Jung, JoonMin; Won, ChongHyun; Chang, SungEun; Choi, JeeHo; Moon, KeeChan (2015). "Clinical and histological patterns of dermatofibroma without gross skin surface change: A comparative study with conventional dermatofibroma". Indian Journal of Dermatology, Venereology, and Leprology. 81 (3): 263. doi:10.4103/0378-6323.154795. ISSN 0378-6323.
- ↑ Mentzel, Thomas; Wiesner, Thomas; Cerroni, Lorenzo; Hantschke, Markus; Kutzner, Heinz; Rütten, Arno; Häberle, Michael; Bisceglia, Michele; Chibon, Frederic; Coindre, Jean-Michel (2012). "Malignant dermatofibroma: clinicopathological, immunohistochemical, and molecular analysis of seven cases". Modern Pathology. 26 (2): 256–267. doi:10.1038/modpathol.2012.157. ISSN 0893-3952.
- ↑ Bernard, J.; Poulalhon, N.; Argenziano, G.; Debarbieux, S.; Dalle, S.; Thomas, L. (2013). "Dermoscopy of dermatofibrosarcoma protuberans: a study of 15 cases". British Journal of Dermatology. 169 (1): 85–90. doi:10.1111/bjd.12318. ISSN 0007-0963.
- ↑ Acosta, Alvaro E.; Vélez, Catalina Santa (2017). "Dermatofibrosarcoma Protuberans". Current Treatment Options in Oncology. 18 (9). doi:10.1007/s11864-017-0498-5. ISSN 1527-2729.
- ↑ Cesarman, Ethel; Damania, Blossom; Krown, Susan E.; Martin, Jeffrey; Bower, Mark; Whitby, Denise (2019). "Kaposi sarcoma". Nature Reviews Disease Primers. 5 (1). doi:10.1038/s41572-019-0060-9. ISSN 2056-676X.
- ↑ Hu, S C-S; Ke, C-L K; Lee, C-H; Wu, C-S; Chen, G-S; Cheng, S-T (2009). "Dermoscopy of Kaposi's sarcoma: Areas exhibiting the multicoloured 'rainbow pattern'". Journal of the European Academy of Dermatology and Venereology. 23 (10): 1128–1132. doi:10.1111/j.1468-3083.2009.03239.x. ISSN 0926-9959.
- ↑ Petter G, Haustein UF (2000). "Histologic subtyping and malignancy assessment of cutaneous squamous cell carcinoma". Dermatol Surg. 26 (6): 521–30. PMID 10848931.
- ↑ Wolberink EA, Pasch MC, Zeiler M, van Erp PE, Gerritsen MJ (2013). "High discordance between punch biopsy and excision in establishing basal cell carcinoma subtype: analysis of 500 cases". J Eur Acad Dermatol Venereol. 27 (8): 985–9. doi:10.1111/j.1468-3083.2012.04628.x. PMID 22759209.
- ↑ Wrone DA, Swetter SM, Egbert BM, Smoller BR, Khavari PA (1996). "Increased proportion of aggressive-growth basal cell carcinoma in the Veterans Affairs population of Palo Alto, California". J Am Acad Dermatol. 35 (6): 907–10. PMID 8959949.
- ↑ Errichetti E, Piccirillo A, Stinco G (2015). "Dermoscopy of prurigo nodularis". J Dermatol. 42 (6): 632–4. doi:10.1111/1346-8138.12844. PMID 25808786.
- ↑ Weigelt N, Metze D, Ständer S (2010). "Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients". J Cutan Pathol. 37 (5): 578–86. doi:10.1111/j.1600-0560.2009.01484.x. PMID 20002240.
- ↑ Witt C, Krengel S (2010). "Clinical and epidemiological aspects of subtypes of melanocytic nevi (Flat nevi, Miescher nevi, Unna nevi)". Dermatol Online J. 16 (1): 1. PMID 20137743.
- ↑ Connolly KL, Giordano C, Dusza S, Busam KJ, Nehal K (2019). "Follicular involvement is frequent in lentigo maligna: Implications for treatment". J Am Acad Dermatol. 80 (2): 532–537. doi:10.1016/j.jaad.2018.07.071. PMC 6333487. PMID 30266559.
- ↑ Connolly KL, Giordano C, Dusza S, Busam KJ, Nehal K (2019). "Follicular involvement is frequent in lentigo maligna: Implications for treatment". J Am Acad Dermatol. 80 (2): 532–537. doi:10.1016/j.jaad.2018.07.071. PMC 6333487. PMID 30266559.
- ↑ Argenziano G, Ferrara G, Francione S, Di Nola K, Martino A, Zalaudek I (2009). "Dermoscopy--the ultimate tool for melanoma diagnosis". Semin Cutan Med Surg. 28 (3): 142–8. doi:10.1016/j.sder.2009.06.001. PMID 19782937.
- ↑ Argenziano G, Soyer HP, Chimenti S, Talamini R, Corona R, Sera F; et al. (2003). "Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet". J Am Acad Dermatol. 48 (5): 679–93. doi:10.1067/mjd.2003.281. PMID 12734496.
- ↑ Menzies, Scott W.; Moloney, Fergal J.; Byth, Karen; Avramidis, Michelle; Argenziano, Giuseppe; Zalaudek, Iris; Braun, Ralph P.; Malvehy, Josep; Puig, Susana; Rabinovitz, Harold S.; Oliviero, Margaret; Cabo, Horacio; Bono, Riccardo; Pizzichetta, Maria A.; Claeson, Magdalena; Gaffney, Daniel C.; Soyer, H. Peter; Stanganelli, Ignazio; Scolyer, Richard A.; Guitera, Pascale; Kelly, John; McCurdy, Olivia; Llambrich, Alex; Marghoob, Ashfaq A.; Zaballos, Pedro; Kirchesch, Herbert M.; Piccolo, Domenico; Bowling, Jonathan; Thomas, Luc; Terstappen, Karin; Tanaka, Masaru; Pellacani, Giovanni; Pagnanelli, Gianluca; Ghigliotti, Giovanni; Ortega, Blanca Carlos; Crafter, Greg; Ortiz, Ana María Perusquía; Tromme, Isabelle; Karaarslan, Isil Kilinc; Ozdemir, Fezal; Tam, Anthony; Landi, Christian; Norton, Peter; Kaçar, Nida; Rudnicka, Lidia; Slowinska, Monika; Simionescu, Olga; Di Stefani, Alessandro; Coates, Elliot; Kreusch, Juergen (2013). "Dermoscopic Evaluation of Nodular Melanoma". JAMA Dermatology. 149 (6): 699. doi:10.1001/jamadermatol.2013.2466. ISSN 2168-6068.
- ↑ Phan A, Dalle S, Touzet S, Ronger-Savlé S, Balme B, Thomas L (2010). "Dermoscopic features of acral lentiginous melanoma in a large series of 110 cases in a white population". Br J Dermatol. 162 (4): 765–71. doi:10.1111/j.1365-2133.2009.09594.x. PMID 19922528.
- ↑ Steglich RB, Meotti CD, Ferreira MS, Lovatto L, de Carvalho AV, de Castro CG (2012). "Dermoscopic clues in the diagnosis of amelanotic and hypomelanotic malignant melanoma". An Bras Dermatol. 87 (6): 920–3. PMC 3699915. PMID 23197217.
- ↑ Witt C, Krengel S (2010). "Clinical and epidemiological aspects of subtypes of melanocytic nevi (Flat nevi, Miescher nevi, Unna nevi)". Dermatol Online J. 16 (1): 1. PMID 20137743.
- ↑ Bauer J, Garbe C (2003). "Acquired melanocytic nevi as risk factor for melanoma development. A comprehensive review of epidemiological data". Pigment Cell Res. 16 (3): 297–306. PMID 12753404.
- ↑ Granter SR, McKee PH, Calonje E, Mihm MC, Busam K (2001). "Melanoma associated with blue nevus and melanoma mimicking cellular blue nevus: a clinicopathologic study of 10 cases on the spectrum of so-called 'malignant blue nevus'". Am J Surg Pathol. 25 (3): 316–23. PMID 11224601.
- ↑ Luo S, Sepehr A, Tsao H (2011). "Spitz nevi and other Spitzoid lesions part I. Background and diagnoses". J Am Acad Dermatol. 65 (6): 1073–84. doi:10.1016/j.jaad.2011.04.040. PMC 3217183. PMID 22082838.
- ↑ Argenziano G, Agozzino M, Bonifazi E, Broganelli P, Brunetti B, Ferrara G; et al. (2011). "Natural evolution of Spitz nevi". Dermatology. 222 (3): 256–60. doi:10.1159/000326109. PMID 21494025.
- ↑ Pedrosa AF, Lopes JM, Azevedo F, Mota A (2016). "Spitz/Reed nevi: a review of clinical-dermatoscopic and histological correlation". Dermatol Pract Concept. 6 (2): 37–41. doi:10.5826/dpc.0602a07. PMC 4866625. PMID 27222770.
- ↑ Tanaka M, Sawada M, Kobayashi K (2011). "Key points in dermoscopic differentiation between lentigo maligna and solar lentigo". J Dermatol. 38 (1): 53–8. doi:10.1111/j.1346-8138.2010.01132.x. PMID 21175756.
- ↑ Sato T, Tanaka M (2014). "Linear sebaceous hyperplasia on the chest". Dermatol Pract Concept. 4 (1): 93–5. doi:10.5826/dpc.0401a16. PMC 3919849. PMID 24520522.
- ↑ Morgan MB, Stevens GL, Switlyk S (2005). "Benign lichenoid keratosis: a clinical and pathologic reappraisal of 1040 cases". Am J Dermatopathol. 27 (5): 387–92. PMID 16148406.
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/5/55/Keratoacanthoma01.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/b/b1/Keratoacanthoma02.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/a/a7/Keratoacanthoma03.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/2/23/Keratoacanthoma13.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/0/03/Keratoacanthoma12.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/6/6b/Keratoacanthoma14.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/3/35/Keratoacanthoma15.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/7/72/Keratoacanthoma19.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/6/67/Keratoacanthoma20.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/8/84/Keratoacanthoma16.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/b/b8/Keratoacanthoma17.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/2/2b/Keratoacanthoma18.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/5/5b/Keratoacanthoma21.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/b/b0/Keratoacanthoma22.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/8/88/Keratoacanthoma40.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/c/c0/Keratoacanthoma23.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/9/98/Keratoacanthoma24.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/3/35/Keratoacanthoma28.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/7/70/Keratoacanthoma29.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/c/c1/Keratoacanthoma30.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/b/b1/Keratoacanthoma31.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/c/c3/Keratoacanthoma32.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/3/32/Keratoacanthoma33.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/d/d9/Keratoacanthoma34.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/d/d5/Keratoacanthoma44.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/3/3d/Keratoacanthoma45.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/b/b3/Keratoacanthoma46.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/0/09/Keratoacanthoma47.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/4/42/Keratoacanthoma48.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/c/ce/Keratoacanthoma49.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/2/24/Keratoacanthoma04.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/e/ee/Keratoacanthoma05.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/0/06/Keratoacanthoma06.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/4/48/Keratoacanthoma07.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/b/b6/Keratoacanthoma41.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/9/9a/Keratoacanthoma25.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/9/9b/Keratoacanthoma26.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/1/17/Keratoacanthoma08.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/9/97/Keratoacanthoma11.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/a/a8/Keratoacanthoma35.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/1/19/Keratoacanthoma36.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/e/e7/Keratoacanthoma42.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/b/b1/Keratoacanthoma09.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/4/42/Keratoacanthoma10.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/6/6d/Keratoacanthoma38.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/4/4d/Keratoacanthoma39.jpg)
![Keratoacanthoma. Adapted from Dermatology Atlas.[1]](/images/f/f2/Keratoacanthoma37.jpg)
![keratoacanthomapendulum. Adapted from Dermatology Atlas.[1]](/images/6/63/Keratoacanthomapendulum01.jpg)
![keratoacanthomapendulum. Adapted from Dermatology Atlas.[1]](/images/2/2c/Keratoacanthomapendulum02.jpg)
![keratoacanthomapendulum. Adapted from Dermatology Atlas.[1]](/images/9/96/Keratoacanthomapendulum03.jpg)
![keratoacanthomapendulum. Adapted from Dermatology Atlas.[1]](/images/0/08/Keratoacanthomapendulum04.jpg)
![keratoacanthomapendulum. Adapted from Dermatology Atlas.[1]](/images/a/ad/Keratoacanthomapendulum05.jpg)
![keratoacanthoma linearepidermalnevus. Adapted from Dermatology Atlas.[1]](/images/7/7a/Keratoacanthoma_linearepidermalnevus01.jpg)
![keratoacanthoma linearepidermalnevus. Adapted from Dermatology Atlas.[1]](/images/4/44/Keratoacanthoma_linearepidermalnevus02.jpg)
![keratoacanthoma linearepidermalnevus. Adapted from Dermatology Atlas.[1]](/images/c/c6/Keratoacanthoma_linearepidermalnevus03.jpg)
![keratoacanthoma linearepidermalnevus. Adapted from Dermatology Atlas.[1]](/images/7/76/Keratoacanthoma_linearepidermalnevus04.jpg)