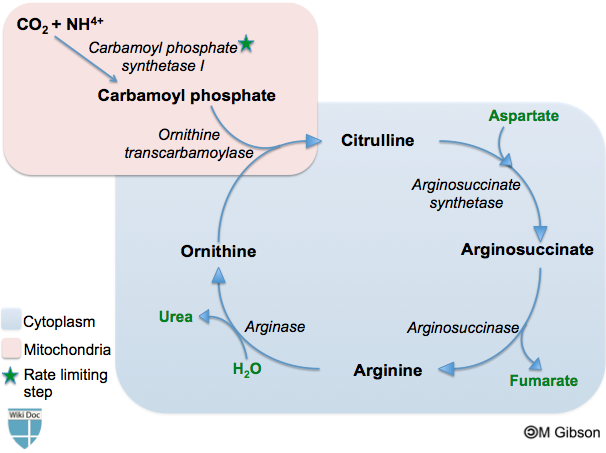
Urea cycle
The urea cycle (also known as the ornithine cycle) is a cycle of biochemical reactions occurring in many animals that produces urea from ammonia (NH3). This cycle was the first metabolic cycle discovered (Krebs and Kurt Henseleit, 1932). In mammals, the urea cycle takes place only in the liver.
Function
Organisms that cannot easily and quickly remove ammonia usually have to convert it to some other substance, like urea or uric acid, which are much less toxic. Insufficiency of the urea cycle occurs in some genetic disorders (inborn errors of metabolism), and in liver failure. The result of liver failure is accumulation of nitrogenous waste, mainly ammonia, which leads to hepatic encephalopathy.
Reactions
The urea cycle consists of five reactions - two mitochondrial and three cytosolic. The cycle converts two amino groups, one from NH4+ and one from Aspartic acid, and a carbon atom from HCO3-, to relatively nontoxic excretion product, urea, at the cost of four "high-energy" phosphate bonds (3 ATP hydrolyzed to 2 ADP and one AMP). Ornithine is the carrier of these carbon and nitrogen atoms.
Reactions of cycle:
| Step | Reactant | Product | Catalyzed by | Location |
| 1 | 2ATP + HCO3- + NH4+ | carbamoyl phosphate + 2ADP + Pi | CPS1 | mitochondria |
| 2 | carbamoyl phosphate + ornithine | citrulline + Pi | OTC | mitochondria |
| 3 | citrulline + aspartate + ATP | argininosuccinate + AMP + PPi | ASS | cytosol |
| 4 | argininosuccinate | Arg + fumarate | ASL | cytosol |
| 5 | Arg + H2O | ornithine + urea | ARG1 | cytosol |
Shown below is an image depicting the different steps of the urea cycle.
Overall energy requirement:
Overall equation of the urea cycle:
Note that reactions related to the urea cycle also causes the reduction of 2 NADH, so the urea cycle releases slightly more energy than it consumes. These NADH are produced in two ways:
- One NADH molecule is reduced by the enzyme glutamate dehydrogenase in the conversion of glutamate to ammonium and α-ketoglutarate. Recall that glutamate is the non-toxic carrier of amine groups. This provides the ammonium ion used in the initial synthesis of carbamoyl phosphate.
- The fumarate released in the cytosol is converted to malate by cytosolic fumarase. This malate is then converted to oxaloacetate by cytosolic malate dehydrogenase, generating a reduced NADH in the cytosol. Oxaloacetate is one of the keto acids preferred by transaminases, and so will be recycled to aspartate, maintained the flow of nitrogen into the urea cycle.
The two NADH produced can provide energy for the formation of 5 ATP, a net production of one high energy phosphate bond for the urea cycle. However, if gluconeogenesis is underway in the cytosol, the latter reducing equivalent is used to drive the reversal of the GAPDH step instead of generating ATP.
The fate of oxaloacetate is either to produce aspartate via oxidative deaminatin or to be converted to phosphoenyl pyruvate, which is a substrate to glucose.()
An excellent way to memorize the Urea Cycle is to remember the phrase "Ordinarily Careless Crappers Are Also Frivolous About Urination." The first letter of each word corresponds to the order in which reactants are combined to give products or intermediates that break apart as one progresses through the cycle.
Regulation
N-Acetylglutamic acid
The synthesis of carbamoyl phosphate and the urea cycle are dependent on the presence of NAcGlu, which allosterically activates CPS1. Synthesis of NAcGlu by NAGS, is stimulated by Arg - allosteric stimulator of NAGS, and Glu - a product in the transamination reactions and one of NAGS's substrates, both of which are elevated when free amino acids are elevated. So, Arg is not only a substrate for the urea cycle reactions but also serves as an activator for the urea cycle.
Substrate concentrations
The remaining enzymes of the cycle are controlled by the concentrations of their substrates. Thus, inherited deficiencies in the cycle enzymes other than ARG1 do not result in significant decrease in urea production (the total lack of any cycle enzyme results in death shortly after birth). Rather, the deficient enzyme's substrate builds up, increasing the rate of the deficient reaction to normal.
The anomalous substrate buildup is not without cost, however. The substrate concentrations become elevated all the way back up the cycle to NH4+, resulting in hyperammonemia (elevated [NH4+]P).
Although the root cause of NH4+ toxicity is not completely understood, a high [NH4+] puts an enormous strain on the NH4+-clearing system, especially in the brain (symptoms of urea cycle enzyme deficiencies include mental retardation and lethargy). This clearing system involves GLUD1 and GLUL, which decrease the 2OG and Glu pools. The brain is most sensitive to the depletion of these pools. Depletion of 2OG decreases the rate of TCAC, whereas Glu is both a neurotransmitter and a precursor to GABA, another neurotransmitter. [1](p.734)
Pathology
Diseases associated with the urea cycle include:
Additional images
-
Urea cycle.
External links
- The chemical logic behind the urea cycle
- Basic Neurochemistry - amino acid disorders
de:Harnstoffzyklus it:Ciclo dell'urea sl:Ciklus sečnine sr:Уреа циклус sv:Ureacykeln Template:WikiDoc Sources