Ornithine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Ornithine is an amino acid, whose structure is:
Role in urea cycle
Ornithine is one of the products of the action of the enzyme arginase on L-arginine, creating urea. Therefore, ornithine is a central part of the urea cycle, which allows for the disposal of excess nitrogen.
Ornithine is not an amino acid coded for by DNA, and in that sense, is not involved in protein synthesis. However, in mammalian non-hepatic tissues, the main use of the urea cycle is in arginine biosynthesis, so as an intermediate in metabolic processes, ornithine is quite important.
Lactamization
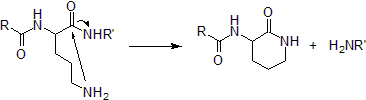
It is believed to not be a part of genetic code because polypeptides containing unprotected ornithines undergo spontaneous lactamization. This proved to be a problem when ornithine was artificially incorporated in 21st amino acid systems.
Other reactions
Ornithine, via the action of ornithine decarboxylase (E.C. 4.1.1.17), is the starting point for the synthesis of polyamines such as putrescine.
In bacteria, such as E. coli, ornithine can be synthesized from L-glutamate.[1]
References
- ↑ "Ornithine Biosynthesis". Retrieved 2007-08-17.