Wilson disease protein: Difference between revisions
m (Robot: Automated text replacement (-{{reflist}} +{{reflist|2}}, -<references /> +{{reflist|2}}, -{{WikiDoc Cardiology Network Infobox}} +)) |
m (→top: bold) |
||
| Line 1: | Line 1: | ||
{{Infobox_gene}} | |||
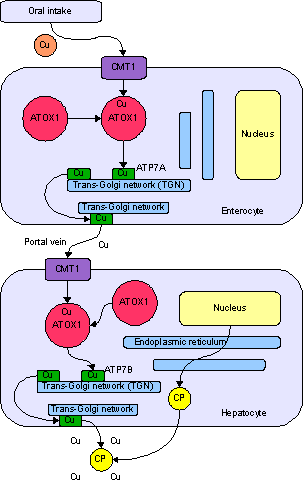
{{ | [[Image:Copper metabolism.png|thumb|Physiological pathway of copper in human body. Cu = [[copper]], CP = [[ceruloplasmin]], ATP7B protein is in [[Hepatocyte]].]] | ||
| | [[File:Structure of ATP7B.jpg|thumb|Simple model of structural feature of ATP7B protein. Cu=Copper binding motif]] | ||
| | |||
| | |||
| | |||
'''Wilson disease protein''' ('''WND'''), also known as '''ATP7B protein''', is a copper-transporting [[P-type ATPase]] which is encoded by the ''ATP7B'' gene. The ATP7B protein is located in the [[trans-Golgi network]] of the liver and brain and balances the copper level in the body by excreting excess copper into bile and plasma. Genetic disorder of the ATP7B gene may cause [[Wilson's disease]], a disease in which copper accumulates in tissues, leading to [[neurological]] or [[psychiatric]] issues and [[liver diseases]]. | |||
'''Wilson disease protein''' (also | |||
== Gene == | |||
[[Wilson disease]] protein is associated with ''ATP7B'' [[gene]],approximate 80 Kb, located on human [[chromosome 13]] and consists of 21 exons.The mRNA transcribed by ''ATP7B'' gene has a size of 7.5 Kb, and which encodes a protein of 1465 [[amino acids]].<ref name =Text2>{{cite journal | vauthors = Terada K, Schilsky ML, Miura N, Sugiyama T | title = ATP7B (WND) protein | journal = The International Journal of Biochemistry & Cell Biology | volume = 30 | issue = 10 | pages = 1063–7 | date = Oct 1998 | pmid = 9785470 | doi = 10.1016/S1357-2725(98)00073-9 }}</ref> | |||
== | The gene is a member of the P-type cation transport [[ATPase]] [[gene family|family]] and encodes a protein with several membrane-spanning domains, an ATPase [[consensus sequence]], a hinge domain, a [[phosphorylation]] site, and at least two putative [[copper]]-binding sites. This protein functions as a monomer, exporting copper out of the cells, such as the efflux of hepatic copper into the [[bile]]. Alternate transcriptional [[splice variant]]s, encoding different isoforms with distinct [[Cell (biology)|cellular]] localizations, have been characterized.<ref>{{cite web | title = Entrez Gene: ATP7B ATPase, Cu++ transporting, beta polypeptide| url = https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=540| accessdate = }}</ref> Wilson disease is caused by various [[mutations]]. One of the common mutations is single base pair mutation,H1069Q.<ref name =Text2 /> | ||
* {{ | |||
== Structure == | |||
ATP7B protein is a copper-transporting [[P-type ATPase]], synthesized as a membrane protein of 165 KDa in human [[hepatoma]] cell line,<ref name =Text2 /> and which is 57% [[homology (biology)|homologous]] to [[menkes disease]] associated protein [[ATP7A]].<ref name =Text1>{{cite journal | vauthors = Harris ED | title = Cellular copper transport and metabolism | journal = Annual Review of Nutrition | volume = 20 | pages = 291–310 | date = 2000 | pmid = 10940336 | doi = 10.1146/annurev.nutr.20.1.291 }}</ref> | |||
ATP7B consists of several [[Protein domain|domains]]: | |||
*Phosphatase domain (TGEA motif Thr-Gly-Glu-Ala)<ref name =Text2 /> | |||
*Phosphorylation domain (DKTGT motif Asp-Lys-Thr-Gly-Thr)<ref name =Text2 /> | |||
*[[ATP-binding motif|ATP binding domain]] (TGDN motif)<ref name =Text2 /> | |||
*Metal binding domain (six Copper binding motifs at the [[N-terminus]] in the [[cytosol]])<ref name =Text2 /> | |||
*Eight [[Transmembrane domain|Transmembrane segments]]<ref name =Text2 /> | |||
The CPC motif (Cys-Pro-Cys) in transmembrane segment 6 characterizes the protein as a heavy metal transporting [[ATPase]].<ref name=book>{{cite book | last1 = Bertini | first1 = Ivano | last2 = Gray | first2 = Harry | last3 = Stiefel | first3 = Edward | last4 = Valentine | first4 = Joan | title = Biological inorganic chemistry:structure and reactivity. | date = 2006 | publisher = University Science Books | location = Sausalito, CA | isbn = 1-891389-43-2 | name-list-format = vanc }}</ref> | |||
The [[copper]] binding motif also shows a high affinity to other transition metal ions like zinc Zn(II), cadmium Cd(II), gold Au(III), and mercury Hg(II). However, copper is able to decrease the zinc binding affinity at low concentration and increase copper binding affinity dramatically with increasing concentration to ensure a strong binding between the motif and copper.<ref name=book /> | |||
As a [[P-type ATPase]]s, ATP7B undergoes auto-[[phosphorylation]] of a key conserved [[aspartic acid]] (D) residue in the DKTGT motif. The ATP binding to the protein initiates the reaction and copper binds to the transmembrane region. Then phosphorylation occurs at the aspartic acid residue in the DKTGT motif with Cu release. Then [[dephosphorylation]] of the aspartic acid residue recovers the protein to ready for the next transport.<ref name= mechanism>{{cite journal | vauthors = Banci L, Bertini I, Cantini F, Ciofi-Baffoni S | title = Cellular copper distribution: a mechanistic systems biology approach | journal = Cellular and Molecular Life Sciences | volume = 67 | issue = 15 | pages = 2563–89 | date = Aug 2010 | pmid = 20333435 | doi = 10.1007/s00018-010-0330-x }}</ref> | |||
== Function == | |||
Most of ATP7B protein is located in the [[trans-Golgi network]] (TGN) of [[hepatocytes]], which is different from its [[homologous protein]] ATP7A.<ref name= Text4 /> Small amount of ATP7B is located in the [[brain]].<ref>{{cite journal | last1=Crisponi |first1=Guido |last2=Nurchi |first2=Valeria Marina |last3=Fanni |first3=Daniela |last4=Gerosa |first4=Clara |last5=Nemolato |first5=Sonia |last6=Faa |first6=Gavino |title = Copper-related diseases: From chemistry to molecular pathology|journal=Coordination Chemistry Reviews|date=April 2010|volume=254|issue=7-8|pages=876–889|doi=10.1016/j.ccr.2009.12.018 | name-list-format = vanc }}</ref> | |||
As a copper-transporting protein, one major function is delivering copper to copper dependent enzymes in [[Golgi apparatus]](e.g.[[holo-ceruloplasmin]](CPN)).<ref name= Text4>{{cite journal | vauthors = Lutsenko S, LeShane ES, Shinde U | title = Biochemical basis of regulation of human copper-transporting ATPases | journal = Archives of Biochemistry and Biophysics | volume = 463 | issue = 2 | pages = 134–48 | date = Jul 2007 | pmid = 17562324 | pmc = 2025638 | doi = 10.1016/j.abb.2007.04.013 }}</ref> | |||
In the human body, [[liver]] plays an important role in copper regulation including removal of extra copper.<ref name= Text4 /> ATP7B participates in the physiological pathway in the copper removal process in two ways: secreting copper into [[blood plasma|plasma]] and excreting copper into [[bile]].<ref name= Text1/> | |||
== Interactions == | |||
=== ATOX1 === | |||
ATP7B receives copper from [[cytosol]]ic protein [[ATOX1|Antioxidant 1 copper chaperone]] (ATOX1).<ref name =Text2 /> This protein targets ATP7B directly in liver in order to transport copper. [[ATOX1]] transfers copper from cytosol to the metal binding domain of ATP7B which control the catalytic activity of ATP7B.<ref name =Text3 /> | |||
Several mutations in ATOX1 can block the copper pathways and cause [[Wilson disease]].<ref name= Text3>{{cite journal | vauthors = Cox DW, Moore SD | title = Copper transporting P-type ATPases and human disease | journal = Journal of Bioenergetics and Biomembranes | volume = 34 | issue = 5 | pages = 333–8 | date = Oct 2002 | pmid = 12539960 | doi=10.1023/A:1021293818125}}</ref> | |||
=== GLRX === | |||
ATP7B interacts with [[Glutaredoxin-1]](GLRX). Subsequent transport is promoted through the reduction of intramolecular [[disulphide bond]]s by GLRX catalysis.<ref name=pmid16884690>{{cite journal | vauthors = Lim CM, Cater MA, Mercer JF, La Fontaine S | title = Copper-dependent interaction of glutaredoxin with the N termini of the copper-ATPases (ATP7A and ATP7B) defective in Menkes and Wilson diseases | journal = Biochem. Biophys. Res. Commun. | volume = 348 | issue = 2 | pages = 428–36 | date = Sep 2006 | pmid = 16884690 | doi = 10.1016/j.bbrc.2006.07.067 }}</ref> | |||
== Associations with Wilson disease == | |||
Wilson disease happens when accumulation of copper inside the liver causes [[mitochondrial damage]] and cell destruction and shows symptoms of [[hepatic disease]]. Then, the loss of excretion of copper in bile leads to an increasing concentration of copper level in urine and causes kidney problems. Therefore, symptoms of Wilson disease could be various including [[kidney disease]] and [[neurological disease]].<ref name =Text3 /> | |||
The major cause is the malfunction of ATP7B<ref name =Text3 /> by single base pair mutations, deletions, frame-shifts, splice errors in ''ATP7B'' gene.<ref name =Text2 /> | |||
== See also == | |||
* [[ATP7A]] and [[Menkes disease]] | |||
* [[Wilson's Disease]] | |||
* [[ATOX1]] | |||
{{Clear}} | |||
== References == | |||
{{reflist|33em}} | |||
== Further reading == | |||
{{refbegin|33em}} | |||
==Further reading== | * {{cite journal | vauthors = Harris ED | title = Cellular copper transport and metabolism. | journal = Annu. Rev. Nutr. | volume = 20 | issue = | pages = 291–310 | year = 2000 | pmid = 10940336 | doi = 10.1146/annurev.nutr.20.1.291 }} | ||
{{refbegin | | * {{cite journal | vauthors = Cox DW, Moore SD | title = Copper transporting P-type ATPases and human disease. | journal = J. Bioenerg. Biomembr. | volume = 34 | issue = 5 | pages = 333–8 | year = 2003 | pmid = 12539960 | doi = 10.1023/A:1021293818125 }} | ||
* {{cite journal | vauthors = Lutsenko S, Efremov RG, Tsivkovskii R, Walker JM | title = Human copper-transporting ATPase ATP7B (the Wilson's disease protein): biochemical properties and regulation. | journal = J. Bioenerg. Biomembr. | volume = 34 | issue = 5 | pages = 351–62 | year = 2003 | pmid = 12539962 | doi = 10.1023/A:1021297919034 }} | |||
* {{cite journal | vauthors = Chappuis P, Bost M, Misrahi M, Duclos-Vallée JC, Woimant F | title = [Wilson disease: clinical and biological aspects] | journal = Ann. Biol. Clin. (Paris) | volume = 63 | issue = 5 | pages = 457–66 | year = 2006 | pmid = 16230279 | doi = }} | |||
*{{cite journal | * {{cite journal | vauthors = La Fontaine S, Mercer JF | title = Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis. | journal = Arch. Biochem. Biophys. | volume = 463 | issue = 2 | pages = 149–67 | year = 2007 | pmid = 17531189 | doi = 10.1016/j.abb.2007.04.021 }} | ||
*{{cite journal | * {{cite journal | vauthors = Lutsenko S, LeShane ES, Shinde U | title = Biochemical basis of regulation of human copper-transporting ATPases. | journal = Arch. Biochem. Biophys. | volume = 463 | issue = 2 | pages = 134–48 | year = 2007 | pmid = 17562324 | pmc = 2025638 | doi = 10.1016/j.abb.2007.04.013 }} | ||
*{{cite journal | * {{cite journal | vauthors = Banci L, Bertini I, Cantini F, Ciofi-Baffoni S | title = Cellular copper distribution: a mechanistic systems biology approach | journal = Cellular and Molecular Life Sciences | volume = 67 | issue = 15 | pages = 2563–89 | date = Aug 2010 | pmid = 20333435 | doi = 10.1007/s00018-010-0330-x }} | ||
*{{cite journal | |||
*{{cite journal | |||
*{{cite journal | |||
}} | |||
{{refend}} | {{refend}} | ||
{{ | == External links == | ||
* [https://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=wilson GeneReviews/NIH/NCBI/UW entry on Wilson Disease or Hepatolenticular Degeneration] | |||
* {{MeshName|Wilson+disease+protein}} | |||
{{PDB Gallery|geneid=540}} | |||
{{ATPases}} | {{ATPases}} | ||
{{ATPase}} | |||
{{Metal metabolism}} | |||
[[ | [[Category:Integral membrane proteins]] | ||
[[Category:Transport proteins]] | |||
Revision as of 05:47, 11 November 2017
| VALUE_ERROR (nil) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Aliases | |||||||
| External IDs | GeneCards: [1] | ||||||
| Orthologs | |||||||
| Species | Human | Mouse | |||||
| Entrez |
|
| |||||
| Ensembl |
|
| |||||
| UniProt |
|
| |||||
| RefSeq (mRNA) |
|
| |||||
| RefSeq (protein) |
|
| |||||
| Location (UCSC) | n/a | n/a | |||||
| PubMed search | n/a | n/a | |||||
| Wikidata | |||||||
| |||||||
Wilson disease protein (WND), also known as ATP7B protein, is a copper-transporting P-type ATPase which is encoded by the ATP7B gene. The ATP7B protein is located in the trans-Golgi network of the liver and brain and balances the copper level in the body by excreting excess copper into bile and plasma. Genetic disorder of the ATP7B gene may cause Wilson's disease, a disease in which copper accumulates in tissues, leading to neurological or psychiatric issues and liver diseases.
Gene
Wilson disease protein is associated with ATP7B gene,approximate 80 Kb, located on human chromosome 13 and consists of 21 exons.The mRNA transcribed by ATP7B gene has a size of 7.5 Kb, and which encodes a protein of 1465 amino acids.[1]
The gene is a member of the P-type cation transport ATPase family and encodes a protein with several membrane-spanning domains, an ATPase consensus sequence, a hinge domain, a phosphorylation site, and at least two putative copper-binding sites. This protein functions as a monomer, exporting copper out of the cells, such as the efflux of hepatic copper into the bile. Alternate transcriptional splice variants, encoding different isoforms with distinct cellular localizations, have been characterized.[2] Wilson disease is caused by various mutations. One of the common mutations is single base pair mutation,H1069Q.[1]
Structure
ATP7B protein is a copper-transporting P-type ATPase, synthesized as a membrane protein of 165 KDa in human hepatoma cell line,[1] and which is 57% homologous to menkes disease associated protein ATP7A.[3]
ATP7B consists of several domains:
- Phosphatase domain (TGEA motif Thr-Gly-Glu-Ala)[1]
- Phosphorylation domain (DKTGT motif Asp-Lys-Thr-Gly-Thr)[1]
- ATP binding domain (TGDN motif)[1]
- Metal binding domain (six Copper binding motifs at the N-terminus in the cytosol)[1]
- Eight Transmembrane segments[1]
The CPC motif (Cys-Pro-Cys) in transmembrane segment 6 characterizes the protein as a heavy metal transporting ATPase.[4]
The copper binding motif also shows a high affinity to other transition metal ions like zinc Zn(II), cadmium Cd(II), gold Au(III), and mercury Hg(II). However, copper is able to decrease the zinc binding affinity at low concentration and increase copper binding affinity dramatically with increasing concentration to ensure a strong binding between the motif and copper.[4]
As a P-type ATPases, ATP7B undergoes auto-phosphorylation of a key conserved aspartic acid (D) residue in the DKTGT motif. The ATP binding to the protein initiates the reaction and copper binds to the transmembrane region. Then phosphorylation occurs at the aspartic acid residue in the DKTGT motif with Cu release. Then dephosphorylation of the aspartic acid residue recovers the protein to ready for the next transport.[5]
Function
Most of ATP7B protein is located in the trans-Golgi network (TGN) of hepatocytes, which is different from its homologous protein ATP7A.[6] Small amount of ATP7B is located in the brain.[7] As a copper-transporting protein, one major function is delivering copper to copper dependent enzymes in Golgi apparatus(e.g.holo-ceruloplasmin(CPN)).[6]
In the human body, liver plays an important role in copper regulation including removal of extra copper.[6] ATP7B participates in the physiological pathway in the copper removal process in two ways: secreting copper into plasma and excreting copper into bile.[3]
Interactions
ATOX1
ATP7B receives copper from cytosolic protein Antioxidant 1 copper chaperone (ATOX1).[1] This protein targets ATP7B directly in liver in order to transport copper. ATOX1 transfers copper from cytosol to the metal binding domain of ATP7B which control the catalytic activity of ATP7B.[8]
Several mutations in ATOX1 can block the copper pathways and cause Wilson disease.[8]
GLRX
ATP7B interacts with Glutaredoxin-1(GLRX). Subsequent transport is promoted through the reduction of intramolecular disulphide bonds by GLRX catalysis.[9]
Associations with Wilson disease
Wilson disease happens when accumulation of copper inside the liver causes mitochondrial damage and cell destruction and shows symptoms of hepatic disease. Then, the loss of excretion of copper in bile leads to an increasing concentration of copper level in urine and causes kidney problems. Therefore, symptoms of Wilson disease could be various including kidney disease and neurological disease.[8] The major cause is the malfunction of ATP7B[8] by single base pair mutations, deletions, frame-shifts, splice errors in ATP7B gene.[1]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Terada K, Schilsky ML, Miura N, Sugiyama T (Oct 1998). "ATP7B (WND) protein". The International Journal of Biochemistry & Cell Biology. 30 (10): 1063–7. doi:10.1016/S1357-2725(98)00073-9. PMID 9785470.
- ↑ "Entrez Gene: ATP7B ATPase, Cu++ transporting, beta polypeptide".
- ↑ 3.0 3.1 Harris ED (2000). "Cellular copper transport and metabolism". Annual Review of Nutrition. 20: 291–310. doi:10.1146/annurev.nutr.20.1.291. PMID 10940336.
- ↑ 4.0 4.1 Bertini I, Gray H, Stiefel E, Valentine J (2006). Biological inorganic chemistry:structure and reactivity. Sausalito, CA: University Science Books. ISBN 1-891389-43-2.
- ↑ Banci L, Bertini I, Cantini F, Ciofi-Baffoni S (Aug 2010). "Cellular copper distribution: a mechanistic systems biology approach". Cellular and Molecular Life Sciences. 67 (15): 2563–89. doi:10.1007/s00018-010-0330-x. PMID 20333435.
- ↑ 6.0 6.1 6.2 Lutsenko S, LeShane ES, Shinde U (Jul 2007). "Biochemical basis of regulation of human copper-transporting ATPases". Archives of Biochemistry and Biophysics. 463 (2): 134–48. doi:10.1016/j.abb.2007.04.013. PMC 2025638. PMID 17562324.
- ↑ Crisponi G, Nurchi VM, Fanni D, Gerosa C, Nemolato S, Faa G (April 2010). "Copper-related diseases: From chemistry to molecular pathology". Coordination Chemistry Reviews. 254 (7–8): 876–889. doi:10.1016/j.ccr.2009.12.018.
- ↑ 8.0 8.1 8.2 8.3 Cox DW, Moore SD (Oct 2002). "Copper transporting P-type ATPases and human disease". Journal of Bioenergetics and Biomembranes. 34 (5): 333–8. doi:10.1023/A:1021293818125. PMID 12539960.
- ↑ Lim CM, Cater MA, Mercer JF, La Fontaine S (Sep 2006). "Copper-dependent interaction of glutaredoxin with the N termini of the copper-ATPases (ATP7A and ATP7B) defective in Menkes and Wilson diseases". Biochem. Biophys. Res. Commun. 348 (2): 428–36. doi:10.1016/j.bbrc.2006.07.067. PMID 16884690.
Further reading
- Harris ED (2000). "Cellular copper transport and metabolism". Annu. Rev. Nutr. 20: 291–310. doi:10.1146/annurev.nutr.20.1.291. PMID 10940336.
- Cox DW, Moore SD (2003). "Copper transporting P-type ATPases and human disease". J. Bioenerg. Biomembr. 34 (5): 333–8. doi:10.1023/A:1021293818125. PMID 12539960.
- Lutsenko S, Efremov RG, Tsivkovskii R, Walker JM (2003). "Human copper-transporting ATPase ATP7B (the Wilson's disease protein): biochemical properties and regulation". J. Bioenerg. Biomembr. 34 (5): 351–62. doi:10.1023/A:1021297919034. PMID 12539962.
- Chappuis P, Bost M, Misrahi M, Duclos-Vallée JC, Woimant F (2006). "[Wilson disease: clinical and biological aspects]". Ann. Biol. Clin. (Paris). 63 (5): 457–66. PMID 16230279.
- La Fontaine S, Mercer JF (2007). "Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis". Arch. Biochem. Biophys. 463 (2): 149–67. doi:10.1016/j.abb.2007.04.021. PMID 17531189.
- Lutsenko S, LeShane ES, Shinde U (2007). "Biochemical basis of regulation of human copper-transporting ATPases". Arch. Biochem. Biophys. 463 (2): 134–48. doi:10.1016/j.abb.2007.04.013. PMC 2025638. PMID 17562324.
- Banci L, Bertini I, Cantini F, Ciofi-Baffoni S (Aug 2010). "Cellular copper distribution: a mechanistic systems biology approach". Cellular and Molecular Life Sciences. 67 (15): 2563–89. doi:10.1007/s00018-010-0330-x. PMID 20333435.
External links
- GeneReviews/NIH/NCBI/UW entry on Wilson Disease or Hepatolenticular Degeneration
- Wilson+disease+protein at the US National Library of Medicine Medical Subject Headings (MeSH)