Unstable angina non ST elevation myocardial infarction complications of bleeding and transfusion
| Unstable angina pectoris | |
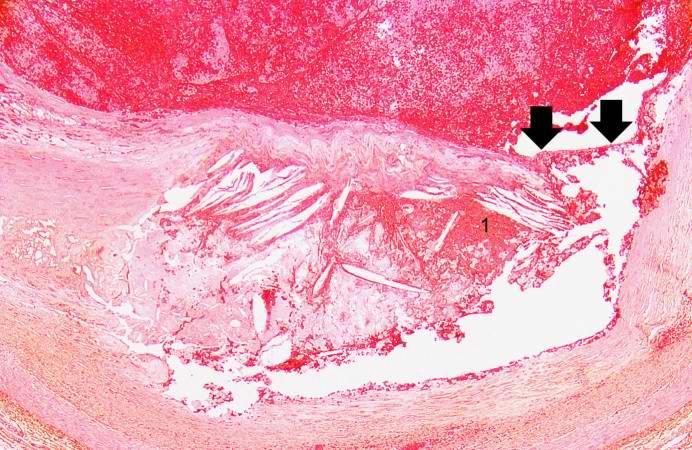 | |
|---|---|
| Plaque rupture in a coronary artery at arrows yielding obstructive thrombus in red. Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology | |
| ICD-10 | I20 |
| ICD-9 | 413 |
| DiseasesDB | 8695 |
| eMedicine | med/133 |
| MeSH | D000787 |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Template:MWT; Cafer Zorkun, M.D., Ph.D. [2]; Varun Kumar, M.B.B.S.; Lakshmi Gopalakrishnan, M.B.B.S.
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [3] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview of Bleeding complications and Blood transfusion associated with Unstable angina/NSTEMI
- Advancements in the efficacy and increased utilization of synergistic anti-platelet agents, anticoagulant therapies, and invasive risk stratification in high-risk patients with unstable angina and non-ST-segment elevation myocardial infarction (NSTEMI) has led to a 40% decrease in mortality from coronary artery disease (CAD) over the preceding 20 years. [1] [2]
- The advanced management of NSTEMI minimizes ischemic events; however the paradigm is that it also increases the risk of bleeding and necessitation for blood transfusion.[3][4] [1] [2]
- Recent analyses and randomized controlled trials demonstrate an independent association between bleeding complications, blood transfusions, and poor outcomes among NSTEMI patients.[5][6] [1] [2]
- Clinical trials of antithrombotic therapies associated with decreased bleeding complications have demonstrated improvements in short-term and long-term survival.
Incidence
- The reported incidence of major bleeding events in the ACS period varies significantly across clinical trials with best estimates between <1 and 14%.[7] [8] [1] [2]
- Differences in individual patient’s propensity to experience a bleeding event may contribute to the observed variance in bleeding incidence for NSTEMI patients. [1] [2]
- The existence of multiple bleeding definitions accounts in part for the disparity of the reported incidence of bleeding complications among NSTEMI patients. [1] [2]
Definitions for bleeding complications
- The two most commonly employed bleeding severity classification schemes are the Thrombolysis In Myocardial Infarction (TIMI) and the Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) scales.[9] [1] [2]
- The GUSTO scale categorizes bleeding as severe or life-threatening, moderate, mild, or none and defines bleeding based on clinical outcomes such as hemodynamic compromise or intracranial hemorrhage.
- The TIMI definition categorizes bleeding as major, minor, minimal, or none and is dependent on changes in laboratory parameters (hemoglobin or hematocrit) and not necessarily on clinically defined events (although intracranial hemorrhage is considered a TIMI major bleed).
- Additional definitions of bleeding events adopt variations of the GUSTO and TIMI classification schemes such as those developed in the SYNERGY,[10] PURSUIT[11] and OASIS-5[12] trials or have adopted trial specific definitions for bleeding complications such as those in FRISC[13], ESSENCE[14], CURE[15] and ACUITY[16] trials.
GUSTO scale:
- Severe or life-threatening: Either intracranial hemorrhage or bleeding that causes hemodynamic compromise and requires intervention
- Moderate: Bleeding that requires blood transfusion but does not result in hemodynamic compromise
- Mild: Bleeding that does not meet criteria for either severe or moderate bleeding
TIMI scale:
- Major: Intracranial hemorrhage or a 5 g/dl decrease in the hemoglobin concentration or a 15% absolute decrease in the hematocrit.
- Minor:
- Observed blood loss: 3 g/dl decrease in the hemoglobin concentration or 10% decrease in the hematocrit
- No observed blood loss: 4 g/dl decrease in the hemoglobin concentration or 12% decrease in the hematocrit
- Minimal: Any clinically overt sign of hemorrhage (including imaging) that is associated with a <3 g/dl decrease in the hemoglobin concentration or <9% decrease in the hematocrit
OASIS-2 trial:
- Major bleeding:
- Life-threatening (fatal, intracranial, requiring surgical intervention or 4 units of blood or plasma expanders)
- Other major bleeding episodes (any event requiring transfusion of 2 or 3 units or judged to be disabling).
- Minor Bleeding: All other bleeding events.
OASIS-5 trial:
- Major Bleeding:
- Fatal, intracranial, retroperitoneal, intraocular leading to vision loss.
- Decrease in Hgb 3 g/dL adjusted for transfusion.
- Transfusion of 2 units.
- Minor Bleeding: Any other clinically significant bleeding not meeting Major criteria leading to study drug interruption, surgery, or transfusion of 1 unit of blood.
CURE trial:
- Major bleeding:
- Life-threatening (fatal, intracranial, requiring surgical intervention, results in hypotension, decrease in Hgb 5 g/dL, or required 4 units of blood).
- Other major bleeding episodes (requiring transfusion of 2 or 3 units, intraocular).
- Minor bleeding: Led to discontinuation of study drug
ACUITY trial:
- Major Bleeding:
- Intracranial or intraocular bleeding.
- Hemorrhage at the access site requiring intervention or hematoma with a diameter of at least 5 cm.
- Hemoglobin decrease of at least 4 g per deciliter without an overt bleeding source or at least 3 g per deciliter with such a source.
- Reoperation for bleeding.
- Transfusion of a blood product.
- Minor Bleeding: not defined
The existence of multiple trial-specific definitions of bleeding events, in addition to TIMI and GUSTO precludes the ability to compare bleeding complication rates across NSTEMI. The variability of pharmacological therapies employed in NSTEMI trials further confounds the ability to clearly define and to determine a single estimate of the incidence of bleeding complications among NTEMI patients.
Predictors and Causes of Bleeding [1] [2]
- Age >75
- Female gender
- Diabetes
- Hypertension
- Anemia
- History of smoking
- History of peripheral arterial disease
- Baseline ST segment deviation > 1mm
- Baseline cardiac biomarker elevation
- Treatment with IV vasopressors in first 24 h
- Pulmonary artery catheter
- Percutaneous coronary intervention
- Intra-aortic balloon pump
- Cardiac catheterization
- History of prior bleeding
- Fibrinolytic therapy
- Treated with unfractionated heparin in first 24 h
- Treated with LMWH in first 24 h
- Treatment with GP IIb/IIIa inhibitors in first 24h
- Glomerular filtration rate <30 mL/min
Blood Transfusions
- Inicidence: 5-10% of NSTEMI patients receive blood transfusions.[17]
- Indication:
- Clinical trial data:
- Yang and colleagues analyzed data from 74,271 NSTEMI/unstable angina patients and found a significant association between blood transfusion and in-hospital mortality.[18]
- Wu and colleagues analyzed 78,974 elderly patients with acute MI and associated the reception of blood transfusion with a significant increased risk of 30-day death when baseline HCT was > 33%.[19]
- Rao and colleagues examined 24,111 NSTEMI patients and found that blood transfusion was associated with a significantly higher risk of 30-day mortality if the nadir HCT was > 24%.[20]
Given this equipoise, it seems reasonable to conclude that routine use of transfusion to maintain arbitrary hemoglobin levels in asymptomatic patients should be avoided.
Prognosis
- Regardless of which bleeding definition is utilized, multiple studies have demonstrated a clear association between bleeding in the NSTEMI population and adverse outcomes including death, stroke, MI and unplanned revascularization.
Clinical trial data:
- Moscucci and colleagues examined the GRACE registry of 24,045 patients with ACS (including unstable angina, NSTEMI, and STEMI) and found an association between GRACE major bleeding and increased in-hospital mortality.[21]
- An analysis by Rao et al.,[22] examined 26,452 ACS-patients enrolled in the PURSUIT, PARAGON B, and GUSTO IIb trials and demonstrated an increased in risk between bleeding severity and 30-day and 6-month death.
- Eikelboom et al.,[23] and Manoukian et. al., [24] have described a similar, significant association between major bleeding in NSTEMI and unstable angina patients and adverse outcomes.
Prognosis between TIMI and GRACE scales:
- The prognostic significance of different bleeding definitions (GUSTO vs TIMI) was assessed by Rao and colleagues.
- Both GUSTO and TIMI bleeding were associated with an increased risk for 30-day death or MI when examined separately. When both definitions were included in the same model, increasing GUSTO bleeding severity was associated with a stepwise increase in the adjusted hazard of death or MI, whereas TIMI bleeding did not correlate with prognosis.
Therefore, bleeding defined by clinical events is likely more important in terms of prognosis rather than bleeding defined solely on the basis of reductions in hemoglobin concentration.
Mechanisms of Bleeding and Increased Mortality:
- Despite the strong association between bleeding and adverse ischemic events, the direct causality of bleeding complications and adverse ischemic events remains uncertain.
1. Anemia and Anemia induced Hemodynamic Compromise:
- During NSTEMI/unstable angina, oxygen delivery to the hypoxic myocardium is normally augmented by a compensatory coronary vasodilatory response.
- Stenotic coronary vessels are devoid of the vasodilatory response and therefore compensatory increases in heart rate and myocardial contractility are employed to maintain systemic oxygen demands.
- The pathophysiology underlying poor outcomes in anemic-NSTEMI patients may be explained by the combination of reduced oxygen delivery to the already hypoxic myocardium and the high myocardial oxygen demand secondary to the compensatory increases in heart rate and stroke volume.
Bleeding therefore, may worsen myocardial ischemia in the NSTEMI patient by inducing a mild anemia and state of tissue hypoperfusion. The subsequent, compensatory tachycardiac and upregulated myocardial contractility state result in a deleterious, myocardial oxygen supply and demand disparity.[25]
2. Potentiation of the inflammatory response:
- Bleeding events precipitate recurrent ischemic events by potentiating the inflammatory response through activation of the platelet and coagulation cascades.
3. Reception of packed red blood cells:
- Mechanistic studies have shown that transfusion of red cells paradoxically does not improve oxygen delivery.[26]
- Stored blood is characterized by an increased affinity for oxygen because of decreased 2,3 DPG levels.
- Stored RBCs have alterations in their morphology and adhesion properties which many speculate increases the risk of vessel occlusion.
- Stored RBCs, devoid of NO produces vasoconstriction, platelet aggregation, and ineffective oxygen delivery.
- Nitric oxide is essential for oxygen uptake into tissues and is the most potent vasodilator.
The synergistic effects of high oxygen affinity and ineffective oxygen delivery of RBC transfusion may account, in part, for poor outcomes among transfused NSTEMI patients.
4. Cessation of antiplatelet and/or antithrombin therapies:
- Analyzing the GRACE database of NSTEMI patients who suffered major bleeding (n=506) within 2 days of admission, Spencer and colleagues discovered that NSTEMI patients with a bleeding event were less likely to have received ASA, thienopyridines, unfractionated heparin or low-molecular-weight heparin during their hospitalization.
- Mortality rates among ACS patients who experienced a major bleeding event were higher if aspirin (OR, 7.55 (95% CI, 4.43 to 12.88)); thienopyridines, (OR, 8.91 (95% CI, 4.39 to 18.12)); and unfractionated heparin, (OR, 1.91 (95% CI, 1.09 to 3.36)) were discontinued, compared to NSTEMI patients who experienced a major bleeding event but continued therapy with these agents.[27]
Prevention
- An optimal NSTEMI/unstable angina management algorithm maximizes the anticoagulant benefit of pharmacological agents, employs coronary intervention when indicated, while simultaneously minimizing bleeding risks.
- Recent trials (OASIS, ACUTIY etc) have correlated reductions in bleeding events with improvements in outcomes of death, MI and stroke.
- Reduction of bleeding complications has therefore become a priority in NSTEMI/unstable angina management.
Alternative means of vascular access for coronary intervention:
Since the introduction of GP IIb/IIIa inhibitors into practice the risk of transfemoral access site bleeding complications has increased by 10%.
- Femoral arteriotomy with femoral head fluoroscopy reduces access site complications.
- Radial artery approach for PCI, is associated with a substantial reduction in bleeding and vascular complications.[28]
- No benefit has been associated with femoral vascular closure devices.
Judicious dosing of antithrombotic and antiplatelet therapies:
- 15% of major bleeding events in NSTEMI/unstable angina patients are preventable with proper bleeding risk assessment and proper administration of anticoagulative agents.
- Data from the CRUSADE registry, reported that 42% of 140,000 NSTEMI patients received at least one excess dose of antithrombotic agent during their hospitalization.[29]. The excess dosing of antithrombotic agents were directly associated with increase rate of bleeding and a prolonged length of hospital stay.
- Risk factors for receiving excessive doses of unfractionated heparin, low-molecular-weight heparin or GP IIb/IIIA inhibitors included:
- Elderly
- Female
- Low body weight
- Diabetes
- Heart failure
- The administration of aspirin and clopidogrel to NSTEMI/unstable angina patients is efficacious and supported by published guidelines.
- The absolute increase in major bleeding is 1% higher with dual antiplatelet therapy compared with aspirin alone.
- In a post hoc analysis of the CURE trial, Peters et al.,[30] described an increased incidence in major bleeding directly associated with aspirin (ASA) dose. (ASA alone: dose 100 mg; 1.9%, 101–199 mg; 2.8%, 200 mg; 3.7%, P=0.0001 ASA+clopidogrel: dose 100 mg; 3.0%, 101–199 mg; 3.4%, 200 mg; 4.9%, P=0.0009).
- Although the ideal dose of aspirin is unknown, these data do suggest that lower doses of aspirin are safer when combined with thienopyridines.
Newer pharmacological strategies to reduce bleeding:
- Bivalirudin:
- REPLACE-2 trial assigned 6,010 patients undergoing urgent or elective PCI to receive unfractionated heaparin with planned GP IIb/IIIa inhibitor or bivalirudin with provisional use of a GP IIb/IIIa inhibitor.[31]
- Composite, 30-day endpoint demonstrated no statistically significant difference in the primary quadruple endpoint of death, MI, target vessel revascularization, or major bleeding between study groups. This was driven by a statistically significant 40% relative risk reduction in major bleeding in patients assigned to bivalirudin.
- ACUITY trial assigned 13,819 moderate-to-high-risk NSTEMI patients to one of three treatment arms: heparin (unfractionated heparin or enoxaparin) with GP IIb/IIIa inhibitor, bivalirudin with GP IIb/IIIa inhibitor, or bivalirudin alone (with provisional use of a GP IIb/IIIa inhibitor).
- Primary endpoint was net clinical benefit at 30-days that consisted of death, MI, ischemia-driven revascularization, or non-CABG major bleeding.
- The bivalirudin-alone strategy was superior to the other two arms (heparin/enoxaparin + GP IIb/IIIa: 11.7%, bivalirudin + GP IIb/IIIa: 11.8%, bivalirudin alone: 10.1%, P < 0.001).
- There were no significant differences in the rates of death, MI, or revascularization between the three arms, but there was a substantial reduction in ACUITY major bleeding among patients assigned to the bivalirudin alone strategy.
- Fondaparinux:
- OASIS-5 trial randomized 20,078 NSTEMI patients to receive fondaparinux or enoxaparin for 6 days.
- Primary outcome of MI, refractory ischemia, or death at 9 days was not statistically different between study arms (5.8 vs. 5.7%, HR 1.01: 95% CI: 0.90–1.13).
- There was however, a significant lower rate of major bleeding at 9 days in patients treated with fondaparinux compared with enoxaparin (2.2 vs. 4.1%, HR 0.52: 95% CI: 0.44–0.61).
- At 30-day follow-up, there was a statistically significant 17% reduction in 30-day mortality among patients treated with fondaparinux versus enoxaparin (2.9 vs. 3.5%, HR 0.83: 95% CI: 0.71–0.97).
- The incidence of catheter-related thrombus was higher among patients assigned to fondaparinux compared with those assigned to enoxparin (1.3% vs. 0.2%), necessitating a protocol amendment that mandated the addition of unfractionated heparin during PCI in patients treated with fondaparinux undergoing coronary intervention.
- OASIS-5 trial randomized 20,078 NSTEMI patients to receive fondaparinux or enoxaparin for 6 days.
Fondaparinux should not be the sole anticoagulant used in patients with ACS undergoing PCI.
Recommendations
- Variability in bleeding definitions across clinical trials makes it difficult to compare the risks of different therapies; however, it is evident that there is an association between bleeding and blood transfusion and an increased risk of adverse events including death, MI, and stroke[1] [2].
- Patients at high risk for bleeding complications, such as the elderly, females, and those with renal dysfunction should be identified as requiring strategies to minimize bleeding risk[1] [2].
- When an invasive strategy is employed, consideration should be given to the use of the radial artery approach[1] [2].
- Prudent dosing of antithrombotic therapy and antiplatelet therapies is essential[1] [2].
- Consideration of agents such as bivalirudin and fondaparinux, which have been shown to reduce ischemic complications while simultaneously reducing bleeding risk for a NSTEMI/unstable angina management scheme seems reasonable[1] [2].
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non–ST-Elevation Myocardial Infarction). Circulation 2007 116: e148 – e304. PMID 17679616
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS. Correction of ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non–ST-Elevation Myocardial Infarction). J Am Coll Cardiol. 2008 Mar 4; 51(9): 974. PMID 17692738
- ↑ Boersma E, Harrington RA, Moliterno DJ, White H, Théroux P, Van de Werf F, de Torbal A, Armstrong PW, Wallentin LC, Wilcox RG, Simes J, Califf RM, Topol EJ, Simoons ML. Platelet glycoproteinIIb/IIIa inhibitors in acute coronary syndromes: a meta-analysis of all major randomised clinical trials. Lancet. 2002;359:189-98
- ↑ James S, Armstrong P, Califf R, Husted S, Kontny F, Niemminen M, Pfisterer M, Simoons ML, Wallentin L. Safety and efficacy of abciximab combined with dalteparin in treatment of acute coronary syndromes. Eur Heart J. 2002;23:1538–45.
- ↑ Rao SV, O'Grady K, Pieper KS, Granger CB, Newby LK, Mahaffey KW, Moliterno DJ, Lincoff AM, Armstrong PW, Van de Werf F, Califf RM, Harrington RA. A comparison of the clinical impact of bleeding measured by two different classifications among patients with acute coronary syndromes. J Am Coll Cardiol. 2006;47:809–16.
- ↑ Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KAA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–82.
- ↑ Popma JJ, Satler LF, Pichard AD, Kent K, Campbell A, Clark C, Merritt A, Leon MB. Vascular complications after balloon and new device angioplasty. Circulation. 1993;88:1569–1578.
- ↑ Stone GW, Grines CL, Cox DA, Garcia E, Tcheng J, Griffin J, Stuckey T, Turco M, Carroll M, Lansky A. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction. N Engl J Med. 2002;346:957–966.
- ↑ Chesebro JH, Knatterud G, Roberts R, Borer J, Cohen LS, Dalen J, Dodge HT, Francis CK, Hillis D, Ludbrook P. Thrombolysis In Myocardial Infarction (TIMI) trial, phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. 1987; 76:142–154.
- ↑ Ferguson JJ, Califf RM, Antman EM, Cohen M, Grines CL, Goodman S, Kereiakes DJ, Langer A, Mahaffey KW, Nessel CC, Armstrong PW, Avezum A, Aylward P, Becker RC, Biasucci L, Borzak S, Col J, Frey MJ, Fry E, Gulba DC, Guneri S, Gurfinkel E, Harrington R, Hochman JS, Kleiman NS, Leon MB, Lopez-Sendon JL, Pepine CJ, Ruzyllo W, Steinhubl SR, Teirstein PS, Toro-Figueroa L, White H; SYNERGY Trial Investigators. Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA. 2004;292:45-54.
- ↑ The PURSUIT Trial Investigators. Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. The PURSUIT Trial Investigators. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy. N Engl J Med. 1998;339:436–43.
- ↑ Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators, Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, Budaj A, Peters RJ, Bassand JP, Wallentin L, Joyner C, Fox KA. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med. 2006;354:1464-76.
- ↑ Fragmin and Fast Revascularization during Instability in Coronary artery disease Investigators, Long-term low-molecular-mass heparin in unstable coronary artery disease: FRISC II prospective randomized multicentre study. Lancet 1999;354:701–707.
- ↑ Cohen M, Demers C, Gurfinkel EP, Turpie AG, Fromell GJ, Goodman S, Langer A, Califf RM, Fox KA, Premmereur J, Bigonzi F. A comparison of low-molecular-weight heparin with unfractionated heparin for unstable coronary artery disease. Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events Study Group. N Engl J Med. 1997;337:447-52.
- ↑ Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494 –502.
- ↑ Stone GW, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, White HD, Pocock SJ, Ware JH, Feit F, Colombo A, Aylward PE, Cequier AR, Darius H, Desmet W, Ebrahimi R, Hamon M, Rasmussen LH, Rupprecht HJ, Hoekstra J, Mehran R, Ohman EM; ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355:2203-16.
- ↑ Sunil V. Rao, Karen Chiswell, Jie-Lena Sun, Christopher B. Granger, L. Kristin Newby, Frans Van de Werf, Harvey D. White, Paul W. Armstong, Robert M. Califf and Robert A. Harrington. International Variation in the Use of Blood Transfusion in Patients With Non–ST-Segment Elevation Acute Coronary Syndromes. Am J Cardiol. 2008;101:25-29.
- ↑ Yang X, Alexander KP, Chen AY, Roe MT, Brindis RG, Rao SV, Gibler WB,Ohman EM, Peterson ED; CRUSADE Investigators. The implications of blood transfusions for patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE National Quality Improvement Initiative. J Am Coll Cardiol. 2005;46:1490–1495.
- ↑ Wu W-C, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230-1236.
- ↑ Rao SV, Jollis JG, Harrington RA, Granger CB, Newby LK, Armstrong PW, Moliterno DJ, Lindblad L, Pieper K, Topol EJ, Stamler JS, Califf RM. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555– 62
- ↑ Moscucci M, Fox KA, Cannon CP, Klein W, López-Sendón J, Montalescot G, White K, Goldberg RJ. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J. 2003;24:1815–23.
- ↑ Rao SV, O'Grady K, Pieper KS, Granger CB, Newby LK, Mahaffey KW, Moliterno DJ, Lincoff AM, Armstrong PW, Van de Werf F, Califf RM, Harrington RA. A comparison of the clinical impact of bleeding measured by two different classifications among patients with acute coronary syndromes. J Am Coll Cardiol. 2006;47:809–16.
- ↑ Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KAA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–82.
- ↑ Manoukian SV, Feit F, Mehran R, Voeltz MD, Ebrahimi R, Hamon M, Dangas GD, Lincoff AM, White HD, Moses JW, King SB 3rd, Ohman EM, Stone GW. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol. 2007;49:1362-8.
- ↑ Sabatine MS, Morrow DA, Giugliano RP, Burton PB, Murphy SA, McCabe CH, Gibson CM, Braunwald E. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042–2049.
- ↑ Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037.
- ↑ Spencer FA, Moscucci M, Granger CB, Gore JM, Goldberg RJ, Steg PG, Goodman SG, Budaj A, Fitzgerald G, Fox KA; for the GRACE Investigators. Does Comorbidity Account for the Excess Mortality in Patients With Major Bleeding in Acute Myocardial Infarction? Circulation. 2007;116:2793-801.
- ↑ Agostoni P, Biondi-Zoccai GG, de Benedictis ML, Rigattieri S, Turri M, Anselmi M, Vassanelli C, Zardini P, Louvard Y, Hamon M. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; Systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 2004;44:349-56.
- ↑ Alexander KP, Chen AY, Roe MT, Newby LK, Gibson CM, Allen-LaPointe NM, Pollack C, Gibler WB, Ohman EM, Peterson ED; CRUSADE Investigators. Excess dosing of antiplatelet and antithrombin agents in the treatment of non-ST-segment elevation acute coronary syndromes. JAMA. 2005;294:3108-16.
- ↑ Peters RJ, Mehta SR, Fox KA, Zhao F, Lewis BS, Kopecky SL, Diaz R, Commerford PJ, Valentin V, Yusuf S; Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) Trial Investigators. Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study. Circulation. 2003;108:1682-7.
- ↑ Lincoff AM, Bittl JA, Harrington RA, Feit F, Kleiman NS, Jackman JD, Sarembock IJ, Cohen DJ, Spriggs D, Ebrahimi R, Keren G, Carr J, Cohen EA, Betriu A, Desmet W, Kereiakes DJ, Rutsch W, Wilcox RG, de Feyter PJ, Vahanian A, Topol EJ, REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 2003;289:853–863.