Typhoid fever primary prevention
|
Typhoid fever Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Typhoid fever primary prevention On the Web |
|
American Roentgen Ray Society Images of Typhoid fever primary prevention |
|
Risk calculators and risk factors for Typhoid fever primary prevention |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aysha Anwar, M.B.B.S[2]
Overview
Primary Prevention
Effective measures for the primary prevention of typhoid fever include the following:
Vaccination
There are two types of vaccines for typhoid fever[1][2][3][4][5][6][7][8]
- Inactivated typhoid vaccine, ViCPS(IM)
- Live typhoid vaccine (oral)
Indications
- Travelers visiting endemic areas.[9][10]
- Health care workers dealing typhoid patients or handling salmonella typhi.
- Individuals at increased risk of acquiring infection such as household contact[11][12]
Contraindications
Following are the contraindications for typhoid vaccine[5][13]
- Severe allergic reaction to the vaccine component
- Severely ill patients or immunocompromised individuals
- Severe reaction to previous dose.
- Children younger than 2 years of age for inactivated vaccine.
- Children younger than six years of age for live attenuated vaccine.
- Pregnancy[14]
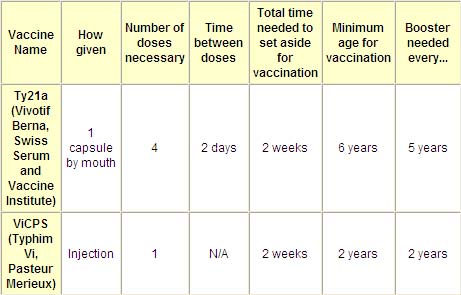
The table below provides the basic information on typhoid vaccines that are available in the United States:
Other measures
Following measures in endemic areas help decrease incidence of typhoid in these areas.[5]
- Improving personal hygiene and sanitation[15]
- Proper sewage disposal.
- Avoiding overcrowding.
- Avoiding close contact or sharing utensils with people already suffering from typhoid.
Following preventive measures can help prevent disease in people traveling to endemic area:[5][16]
- Avoiding foods and beverages from street vendors.
- Avoiding raw fruits and vegetables that cannot be peeled.
- Avoid consumption of ice cream
- Eating food which is properly cooked and served hot.
- Using hard cooked eggs
- Using pasteurized milk products.
- Using boiled or bottled water.
References
- ↑ Schmoldt A, Benthe HF, Haberland G (1975). "Digitoxin metabolism by rat liver microsomes". Biochem Pharmacol. 24 (17): 1639–41. PMID http://dx.doi.org/10.1136/bmj.316.7125.110 Check
|pmid=value (help). - ↑ Wahdan, M. H., et al. "A controlled field trial of live Salmonella typhi strain Ty 21a oral vaccine against typhoid: three-year results." Journal of Infectious Diseases 145.3 (1982): 292-295.
- ↑ Acharya IL, Lowe CU, Thapa R, Gurubacharya VL, Shrestha MB, Cadoz M; et al. (1987). "Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi. A preliminary report". N Engl J Med. 317 (18): 1101–4. doi:10.1056/NEJM198710293171801. PMID 3657877.
- ↑ Lin FY, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC; et al. (2001). "The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children". N Engl J Med. 344 (17): 1263–9. doi:10.1056/NEJM200104263441701. PMID 11320385.
- ↑ 5.0 5.1 5.2 5.3 Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ (2002). "Typhoid fever". N Engl J Med. 347 (22): 1770–82. doi:10.1056/NEJMra020201. PMID 12456854.
- ↑ MacFadden DR, Bogoch II, Andrews JR (2016). "Advances in diagnosis, treatment, and prevention of invasive Salmonella infections". Curr Opin Infect Dis. doi:10.1097/QCO.0000000000000302. PMID 27479027.
- ↑ http://wwwnc.cdc.gov/travel/diseases/typhoid
- ↑ Hainsworth T (2002). "Travel vaccines: a guide to appropriate use". Nurs Times. 98 (25): 40–2. PMID 12168224.
- ↑ John TJ (1995). "Typhoid vaccine". Indian Pediatr. 32 (3): 391–3. PMID 8613306.
- ↑ http://www.cdc.gov/vaccines/hcp/vis/vis-statements/typhoid.html
- ↑ Jackson BR, Iqbal S, Mahon B, Centers for Disease Control and Prevention (CDC) (2015). "Updated recommendations for the use of typhoid vaccine--Advisory Committee on Immunization Practices, United States, 2015". MMWR Morb Mortal Wkly Rep. 64 (11): 305–8. PMID 25811680.
- ↑ Steinberg EB, Bishop R, Haber P, Dempsey AF, Hoekstra RM, Nelson JM; et al. (2004). "Typhoid fever in travelers: who should be targeted for prevention?". Clin Infect Dis. 39 (2): 186–91. doi:10.1086/421945. PMID 15307027.
- ↑ http://www.cdc.gov/vaccines/hcp/vis/vis-statements/typhoid.html
- ↑ Galev A, Nacheva A (2014). "[Pregnancy and vaccinoprevention]". Akush Ginekol (Sofiia). 53 (1): 51–6. PMID 24919344.
- ↑ Ivanoff, Bernard. "Typhoid fever: global situation and WHO recommendations." Southeast Asian Journal of Tropical Medicine and Public Health 26 (1995): 1-6.
- ↑ http://wwwnc.cdc.gov/travel/diseases/typhoid