Typhlitis
|
WikiDoc Resources for Typhlitis |
|
Articles |
|---|
|
Most recent articles on Typhlitis |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Typhlitis at Clinical Trials.gov Clinical Trials on Typhlitis at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Typhlitis
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Typhlitis Discussion groups on Typhlitis Directions to Hospitals Treating Typhlitis Risk calculators and risk factors for Typhlitis
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Typhlitis |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Synonyms and keywords: neutropenic colitis; neutropenic enterocolitis; cecitis
Overview
Typhlitis occurs in neutropenic patients undergoing treatment for a malignancy, most frequently patients with acute leukemia who are receiving chemotherapy. It has also been reported in patients with aplastic anemia, lymphoma, or acquired immunodeficiency syndrome and after kidney transplantation. Typhlitis is characterized by edema and inflammation of the cecum, the ascending colon, and sometimes the terminal ileum. The inflammation can be so severe that transmural necrosis, perforation, and death can result. The mechanism of the condition is not known, but it is probably due to a combination of ischemia, infection (especially with cytomegalovirus), mucosal hemorrhage, and perhaps neoplastic infiltration. Treatment consists of bowel rest, total parenteral nutrition, antibiotics, and aggressive fluid and electrolyte replacement.
Historical Perspective
The word Typhlitis comes from the Greek typhi, meaning "blind" in reference to the blind-ending cecum, and the cecum can be affected by neutropenic enterocolitis, which can also damage the ileum and progress to the ascending colon.[1]
Classification
There is no established system for the classification of typhlitis.
Pathophysiology
- The exact pathogenesis of neutropenic enterocolitis is not fully understood.
- Intestinal mucosal damage, neutropenia, and the immunocompromised status of the afflicted patients appear to be the key factors in disease initiation.
- Intestinal edema, engorged veins, and a disturbed mucosal surface result from these early circumstances, making the mucosa more prone to bacterial intramural invasion.
- The intestinal motility is affected by the distension and necrosis caused directly by chemotherapeutic agents.
- Superimposed infections caused by bacteria,fungi and viruses can also disrupts the already damaged mucosa leading further intestinal edema,distension and necrosis of intestinal layer which lead to intestinal perforation.[2]
- Gram-negative rods, gram-positive cocci, enterococci, fungi, and viruses have all been blamed for the outbreak.[3][4]
Causes by Organ System
| Cardiovascular | No underlying causes |
| Chemical/Poisoning | No underlying causes |
| Dental | No underlying causes |
| Dermatologic | No underlying causes |
| Drug Side Effect | Doxorubicin Hydrochloride, Sulfasalazine |
| Ear Nose Throat | No underlying causes |
| Endocrine | No underlying causes |
| Environmental | No underlying causes |
| Gastroenterologic | No underlying causes |
| Genetic | No underlying causes |
| Hematologic | No underlying causes |
| Iatrogenic | No underlying causes |
| Infectious Disease | No underlying causes |
| Musculoskeletal/Orthopedic | No underlying causes |
| Neurologic | No underlying causes |
| Nutritional/Metabolic | No underlying causes |
| Obstetric/Gynecologic | No underlying causes |
| Oncologic | No underlying causes |
| Ophthalmologic | No underlying causes |
| Overdose/Toxicity | No underlying causes |
| Psychiatric | No underlying causes |
| Pulmonary | No underlying causes |
| Renal/Electrolyte | No underlying causes |
| Rheumatology/Immunology/Allergy | No underlying causes |
| Sexual | No underlying causes |
| Trauma | No underlying causes |
| Urologic | No underlying causes |
| Miscellaneous | No underlying causes |
Causes in Alphabetical Order
Differentiating Typhlitis from other Diseases
Typhlitis must be distinguished from other diseases characterized by fever, abdominal pain, and diarrhea. Before diagnosing this condition, some diseases with similar clinical manifestations should be ruled out.
- Clostridium difficile infection[5]
- Cytomegalovirus colitis[6]
- Norovirus infection[7]
- Graft versus host disease[8]
- Acute appendicitis[9]
- Ischemic colitis[10]
- Ogilvie syndrome (colonic pseudo-obstruction)[11]
Epidemiology and Demographics
The prevalence of neutropenic enterocolitis varies between studies. Gorschlüter et al. conducted a systematic review and found that the incidence rate from 21 studies was 5.3 percent in patients hospitalized for hematological malignancies, high-dose chemotherapy for solid tumors, or aplastic anemia. Another cohort study discovered it in 3.5% of 317 severely neutropenic patients. The prevalence of neutropenic enterocolitis has been increasing in tandem with the increased use of chemotherapy, especially the agents known for causing mucositis.[12][13]
Patients with hematologic malignancies are more likely to develop neutropenic enterocolitis as a result of their underlying malignancy as well as their treatment regimens. Neutropenic enterocolitis has also been reported in patients taking immunosuppressive medications, patients diagnosed with solid tumors and autoimmune conditions.[14]
Risk Factors
The most potent risk factor in the development of [disease name] is [risk factor 1]. Other risk factors include [risk factor 2], [risk factor 3], and [risk factor 4].
OR
Common risk factors in the development of [disease name] include [risk factor 1], [risk factor 2], [risk factor 3], and [risk factor 4].
OR
Common risk factors in the development of [disease name] may be occupational, environmental, genetic, and viral.
Screening
There is insufficient evidence to recommend routine screening for [disease/malignancy].
OR
According to the [guideline name], screening for [disease name] is not recommended.
OR
According to the [guideline name], screening for [disease name] by [test 1] is recommended every [duration] among patients with [condition 1], [condition 2], and [condition 3].
Natural History, Complications, and Prognosis
If left untreated, [#]% of patients with [disease name] may progress to develop [manifestation 1], [manifestation 2], and [manifestation 3].
OR
Common complications of [disease name] include [complication 1], [complication 2], and [complication 3].
OR
Prognosis is generally excellent/good/poor, and the 1/5/10-year mortality/survival rate of patients with [disease name] is approximately [#]%.
Diagnosis
Diagnostic Study of Choice
The diagnosis of [disease name] is made when at least [number] of the following [number] diagnostic criteria are met: [criterion 1], [criterion 2], [criterion 3], and [criterion 4].
OR
The diagnosis of [disease name] is based on the [criteria name] criteria, which include [criterion 1], [criterion 2], and [criterion 3].
OR
The diagnosis of [disease name] is based on the [definition name] definition, which includes [criterion 1], [criterion 2], and [criterion 3].
OR
There are no established criteria for the diagnosis of [disease name].
History and Symptoms
The majority of patients with [disease name] are asymptomatic.
OR
The hallmark of [disease name] is [finding]. A positive history of [finding 1] and [finding 2] is suggestive of [disease name]. The most common symptoms of [disease name] include [symptom 1], [symptom 2], and [symptom 3]. Common symptoms of [disease] include [symptom 1], [symptom 2], and [symptom 3]. Less common symptoms of [disease name] include [symptom 1], [symptom 2], and [symptom 3].
Physical Examination
Patients with [disease name] usually appear [general appearance]. Physical examination of patients with [disease name] is usually remarkable for [finding 1], [finding 2], and [finding 3].
OR
Common physical examination findings of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
The presence of [finding(s)] on physical examination is diagnostic of [disease name].
OR
The presence of [finding(s)] on physical examination is highly suggestive of [disease name].
Laboratory Findings
An elevated/reduced concentration of serum/blood/urinary/CSF/other [lab test] is diagnostic of [disease name].
OR
Laboratory findings consistent with the diagnosis of [disease name] include [abnormal test 1], [abnormal test 2], and [abnormal test 3].
OR
[Test] is usually normal among patients with [disease name].
OR
Some patients with [disease name] may have elevated/reduced concentration of [test], which is usually suggestive of [progression/complication].
OR
There are no diagnostic laboratory findings associated with [disease name].
Electrocardiogram
There are no ECG findings associated with [disease name].
OR
An ECG may be helpful in the diagnosis of [disease name]. Findings on an ECG suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
X-ray
There are no x-ray findings associated with [disease name].
OR
An x-ray may be helpful in the diagnosis of [disease name]. Findings on an x-ray suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
There are no x-ray findings associated with [disease name]. However, an x-ray may be helpful in the diagnosis of complications of [disease name], which include [complication 1], [complication 2], and [complication 3].
Echocardiography or Ultrasound
There are no echocardiography/ultrasound findings associated with [disease name].
OR
Echocardiography/ultrasound may be helpful in the diagnosis of [disease name]. Findings on an echocardiography/ultrasound suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
There are no echocardiography/ultrasound findings associated with [disease name]. However, an echocardiography/ultrasound may be helpful in the diagnosis of complications of [disease name], which include [complication 1], [complication 2], and [complication 3].
CT
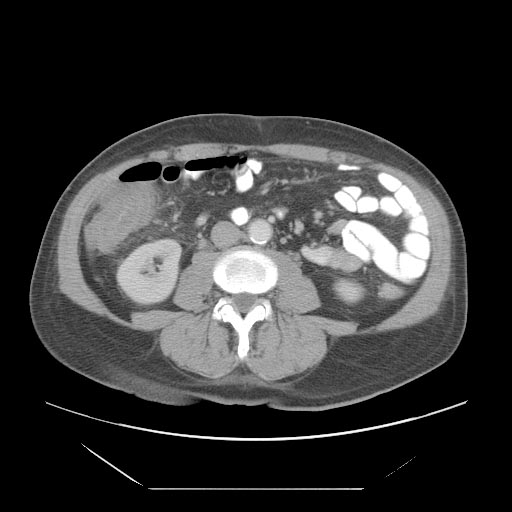
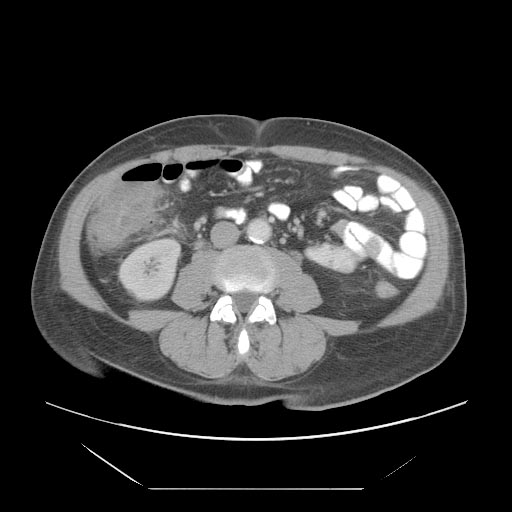
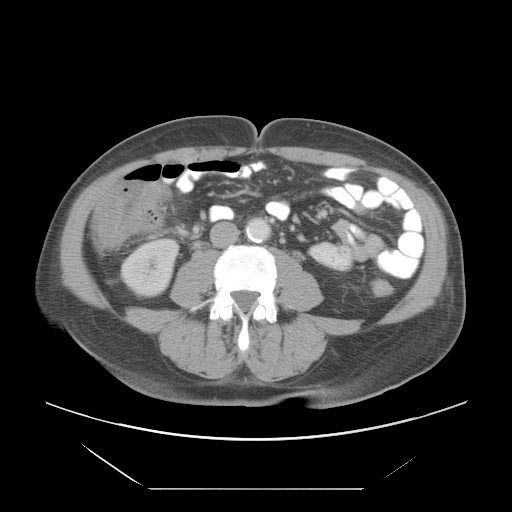
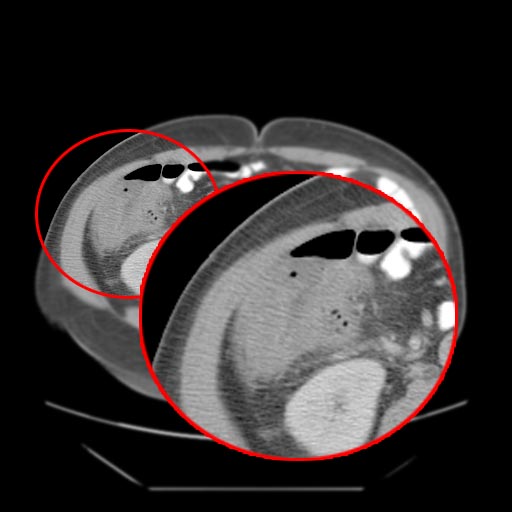
- Cecal distention and circumferential thickening of the cecal wall
- Inflammatory stranding of the adjacent mesenteric fat is a common finding.
- Detection of complications such as pneumatosis, pneumoperitoneum, and pericolic fluid collections is important because they indicate a need for urgent surgical management.
Causes
Life Threatening Causes
Life-threatening causes include conditions which may result in death or permanent disability within 24 hours if left untreated.
MRI
There are no MRI findings associated with [disease name].
OR
[Location] MRI may be helpful in the diagnosis of [disease name]. Findings on MRI suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
There are no MRI findings associated with [disease name]. However, a MRI may be helpful in the diagnosis of complications of [disease name], which include [complication 1], [complication 2], and [complication 3].
Other Imaging Findings
There are no other imaging findings associated with [disease name].
OR
[Imaging modality] may be helpful in the diagnosis of [disease name]. Findings on an [imaging modality] suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
Other Diagnostic Studies
There are no other diagnostic studies associated with [disease name].
OR
[Diagnostic study] may be helpful in the diagnosis of [disease name]. Findings suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
Other diagnostic studies for [disease name] include [diagnostic study 1], which demonstrates [finding 1], [finding 2], and [finding 3], and [diagnostic study 2], which demonstrates [finding 1], [finding 2], and [finding 3].
Treatment
Medical Therapy
- 1. Community-acquired infection in adults [15]
- 1.1. Mild-to-moderate severity (perforated or abscessed appendicitis and other infections of mild-to-moderate severity):
- 1.1.1. Single agent:
- Preferred regimen (1): Cefoxitin 2 g IV q6h
- Preferred regimen (2): Ertapenem 1 g IV q24h
- Preferred regimen (3): Moxifloxacin 400 mg IV q24h
- Preferred regimen (4): Tigecycline 100 mg initial dose, THEN 50 mg IV q12h
- Preferred regimen (5): Ticarcillin-clavulanic acid 3.1 g IV q6h; FDA labeling indicates 200 mg/kg/day in divided doses every 6 h for moderate infection
- 1.1.2. Combination:
- Preferred regimen (1): Cefazolin 1–2 g IV q8h AND Metronidazole 500 mg IV q8–12 h OR 1500 mg q24h
- Preferred regimen (2): Cefuroxime 1.5 g IV q8h AND Metronidazole 500 mg IV q8–12 h OR 1500 mg q24h
- Preferred regimen (3): Ceftriaxone 1–2 g IV q12–24 h AND Metronidazole 500 mg IV q8–12 h OR 1500 mg q24h
- Preferred regimen (4): Cefotaxime 1–2 g IV q6–8 h AND Metronidazole 500 mg IV q8–12 h OR 1500 mg q24h
- Preferred regimen (5): Ciprofloxacin 400 mg IV q12h AND Metronidazole 500 mg IV q8–12 h OR 1500 mg q24h
- Preferred regimen (6): Levofloxacin 750 mg IV q24h AND Metronidazole 500 mg IV q8–12 h OR 1500 mg q24h
- 1.2. High risk or severity (severe physiologic disturbance, advanced age, or immunocompromised state):
- 1.2.1. Single agent:
- Preferred regimen (1): Imipenem-cilastatin 500 mg IV q6h OR 1 g q8h
- Preferred regimen (2): Meropenem 1 g IV q8h
- Preferred regimen (3): Doripenem 500 mg IV q8h
- Preferred regimen (4): Piperacillin-tazobactam 3.375 g IV q6h
- 1.2.2. Combination:
- Preferred regimen (1): Cefepime 2 g q8–12 h AND Metronidazole 500 mg IV q8–12 h or 1500 mg q24h
- Preferred regimen (2): Ceftazidime 2 g q8h AND Metronidazole 500 mg IV q8–12 h or 1500 mg q24h
- Preferred regimen (3): Ciprofloxacin 400 mg q12h AND Metronidazole 500 mg IV q8–12 h or 1500 mg q24h
- Preferred regimen (4): Levofloxacin 750 mg q24h AND Metronidazole 500 mg IV q8–12 h or 1500 mg q24h
- Note: Antimicrobial therapy of established infection should be limited to 4–7 days, unless it is difficult to achieve adequate source control. Longer durations of therapy have not been associated with improved outcome.
- 2. Health Care–Associated Complicated Intra-abdominal Infection [15]
- 2.1. Less than 20% Resistant Pseudomonas aeruginosa, Extended-spectrum B-lactamase-producing Enterobacteriaceae, Acinetobacter, or other multidrug resistant gram-negative bacilli:
- Preferred regimen (1): Meropenem 1 g IV q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Ceftazidime 2 g IV q8h AND Metronidazole 500 mg q8–12 h or 1500 mg q24h
- Preferred regimen (2): Imipenem-cilastatin 500 mg IV 6 h OR 1 g q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Ceftazidime 2 g IV q8h AND Metronidazole 500 mg IV q8–12 h or 1500 mg q24h
- Preferred regimen (3): Doripenem 500 mg IV q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Ceftazidime 2 g IV q8h AND Metronidazole 500 mg IV every 8–12 h or 1500 mg q24h
- Preferred regimen (4): Meropenem 1 g IV q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Cefepime 2 g IV q8–12 h AND Metronidazole 500 mg q8–12 h or 1500 mg q24h
- Preferred regimen (5): Imipenem-cilastatin 500 mg IV q6h OR 1 g q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Cefepime 2 g IV q8–12 h AND Metronidazole 500 mg IV q8–12 h or 1500 mg q24h
- Preferred regimen (6): Doripenem 500 mg IV q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Cefepime 2 g IV q8–12 h AND Metronidazole 500 mg IV q8–12 h or 1500 mg q24h
- 2.2. Extended-spectrum B-lactamase-producing Enterobacteriaceae:
- Preferred regimen (1): Meropenem 1 g IV q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Gentamicin 5–7 mg/kg IV q24h
- Preferred regimen (2): Meropenem 1 g IV q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Tobramycin 5–7 mg/kg IV q24h
- Preferred regimen (3): Meropenem 1 g IV q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Amikacin 15–20 mg/kg IV q24h
- Preferred regimen (4): Imipenem-cilastatin 500 mg IV q6h OR 1 g q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Gentamicin 5–7 mg/kg IV q24h
- Preferred regimen (5): Imipenem-cilastatin 500 mg IV q6h OR 1 g q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Tobramycin 5–7 mg/kg IV q24h
- Preferred regimen (6): Imipenem-cilastatin 500 mg IV q6h OR 1 g q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Amikacin 15–20 mg/kg IV q24h
- Preferred regimen (7): Doripenem 500 mg IV q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Gentamicin 5–7 mg/kg IV q24h
- Preferred regimen (8): Doripenem 500 mg IV q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Tobramycin 5–7 mg/kg IV q24h
- Preferred regimen (9): Doripenem 500 mg IV q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Amikacin 15–20 mg/kg IV q24h
- 2.3. Pseudomonas aeruginosa with more than 20% resistant to ceftazidime:
- Preferred regimen (1): Meropenem 1 g IV q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Gentamicin 5–7 mg/kg IV q24h
- Preferred regimen (2): Meropenem 1 g IV q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Tobramycin 5–7 mg/kg IV q24h
- Preferred regimen (3): Meropenem 1 g IV q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Amikacin 15–20 mg/kg IV q24h
- Preferred regimen (4): Imipenem-cilastatin 500 mg IV q6h OR 1 g q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Gentamicin 5–7 mg/kg IV q24h
- Preferred regimen (5): Imipenem-cilastatin 500 mg IV q6h OR 1 g q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Tobramycin 5–7 mg/kg IV q24h
- Preferred regimen (6): Imipenem-cilastatin 500 mg IV q6h OR 1 g q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Amikacin 15–20 mg/kg IV q24h
- Preferred regimen (7): Doripenem 500 mg IV q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Gentamicin 5–7 mg/kg IV q24h
- Preferred regimen (8): Doripenem 500 mg IV q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Tobramycin 5–7 mg/kg IV q24h
- Preferred regimen (9): Doripenem 500 mg IV q8h AND Piperacillin-tazobactam 3.375 g IV q6h AND Amikacin 15–20 mg/kg IV q24h
- 2.4.Methicillin-resistant Staphylococcus aureus (MRSA):
- Preferred regimen: Vancomycin 15–20 mg/kg IV q8–12 h
- Note: Antimicrobial therapy of established infection should be limited to 4–7 days, unless it is difficult to achieve adequate source control. Longer durations of therapy have not been associated with improved outcome.
- 3. Community-acquired infection in pediatric patients[15]
- 3.1. Single agent:
- Preferred regimen (1): Ertapenem 3 months to 12 years 15 mg/kg bid (not to exceed 1 g/day) Every 12 h, older than 13 years 1 g/day Every 24 h OR
- Preferred regimen (2): Meropenem 60 mg/kg/day q8h
- Preferred regimen (3): Imipenem-cilastatin 60–100 mg/kg/day IV q6h
- Preferred regimen (4): Ticarcillin-clavulanate 200–300 mg/kg/day IV of ticarcillin component q4–6 h
- Preferred regimen (5): Piperacillin-tazobactam 200–300 mg/kg/day IV of piperacillin component q6–8 h
- 3.2.Combination:
- Preferred regimen(1): Ceftriaxone 50–75 mg/kg/day q12–24 h, AND Metronidazole 30–40 mg/kg/day q8h
- Preferred regimen(2): Cefotaxime 150–200 mg/kg/day q6–8 h, AND Metronidazole 30–40 mg/kg/day q8h
- Preferred regimen(3): Cefepime 100 mg/kg/day q12h, AND Metronidazole 30–40 mg/kg/day q8h
- Preferred regimen(4): Ceftazidime 150 mg/kg/day q8 h, AND Metronidazole 30–40 mg/kg/day q8h
- Preferred regimen(5): Gentamicin 3–7.5 mg/kg/day q2–4 h, AND Metronidazole 30–40 mg/kg/day q8h ± Ampicillin 200 mg/kg/day q6h
- Preferred regimen(6): Gentamicin 3–7.5 mg/kg/day q2–4 h, AND Clindamycin 20–40 mg/kg/day q6–8 h ± Ampicillin 200 mg/kg/day q6h
- Preferred regimen(7): Tobramycin 3.0–7.5 mg/kg/day q8–24 h, AND Metronidazole 30–40 mg/kg/day q8h ± Ampicillin 200 mg/kg/day q6h
- Preferred regimen(8): Tobramycin 3.0–7.5 mg/kg/day q8–24 h, AND Clindamycin 20–40 mg/kg/day q6–8 h ± Ampicillin 200 mg/kg/day q6h
- Note: Antimicrobial therapy of established infection should be limited to 4–7 days, unless it is difficult to achieve adequate source control. Longer durations of therapy have not been associated with improved outcome.
References
- ↑ Ullery BW, Pieracci FM, Rodney JR, Barie PS (2009). "Neutropenic enterocolitis". Surg Infect (Larchmt). 10 (3): 307–14. doi:10.1089/sur.2008.061. PMID 19566419.
- ↑ Cloutier RL (2009). "Neutropenic enterocolitis". Emerg Med Clin North Am. 27 (3): 415–22. doi:10.1016/j.emc.2009.04.002. PMID 19646645.
- ↑ Rodrigues FG, Dasilva G, Wexner SD (2017). "Neutropenic enterocolitis". World J Gastroenterol. 23 (1): 42–47. doi:10.3748/wjg.v23.i1.42. PMC 5221285. PMID 28104979.
- ↑ "StatPearls". ( ). 2021: . PMID 31869058.
- ↑ Czepiel J, Dróżdż M, Pituch H, Kuijper EJ, Perucki W, Mielimonka A; et al. (2019). "Clostridium difficile infection: review". Eur J Clin Microbiol Infect Dis. 38 (7): 1211–1221. doi:10.1007/s10096-019-03539-6. PMC 6570665 Check
|pmc=value (help). PMID 30945014. - ↑ Pillet S, Pozzetto B, Roblin X (2016). "Cytomegalovirus and ulcerative colitis: Place of antiviral therapy". World J Gastroenterol. 22 (6): 2030–45. doi:10.3748/wjg.v22.i6.2030. PMC 4726676. PMID 26877608.
- ↑ "StatPearls". 2021. PMID 31335045.
- ↑ Ramachandran V, Kolli SS, Strowd LC (2019). "Review of Graft-Versus-Host Disease". Dermatol Clin. 37 (4): 569–582. doi:10.1016/j.det.2019.05.014. PMID 31466596.
- ↑ Bhangu A, Søreide K, Di Saverio S, Assarsson JH, Drake FT (2015). "Acute appendicitis: modern understanding of pathogenesis, diagnosis, and management". Lancet. 386 (10000): 1278–1287. doi:10.1016/S0140-6736(15)00275-5. PMID 26460662.
- ↑ Theodoropoulou A, Koutroubakis IE (2008). "Ischemic colitis: clinical practice in diagnosis and treatment". World J Gastroenterol. 14 (48): 7302–8. doi:10.3748/wjg.14.7302. PMC 2778113. PMID 19109863.
- ↑ Nesher L, Rolston KV (2013). "Neutropenic enterocolitis, a growing concern in the era of widespread use of aggressive chemotherapy". Clin Infect Dis. 56 (5): 711–7. doi:10.1093/cid/cis998. PMID 23196957.
- ↑ Gorschlüter M, Mey U, Strehl J, Ziske C, Schepke M, Schmidt-Wolf IG; et al. (2005). "Neutropenic enterocolitis in adults: systematic analysis of evidence quality". Eur J Haematol. 75 (1): 1–13. doi:10.1111/j.1600-0609.2005.00442.x. PMID 15946304.
- ↑ Aksoy DY, Tanriover MD, Uzun O, Zarakolu P, Ercis S, Ergüven S; et al. (2007). "Diarrhea in neutropenic patients: a prospective cohort study with emphasis on neutropenic enterocolitis". Ann Oncol. 18 (1): 183–189. doi:10.1093/annonc/mdl337. PMID 17023562.
- ↑ Nesher L, Rolston KV (2013). "Neutropenic enterocolitis, a growing concern in the era of widespread use of aggressive chemotherapy". Clin Infect Dis. 56 (5): 711–7. doi:10.1093/cid/cis998. PMID 23196957.
- ↑ 15.0 15.1 15.2 Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ; et al. (2010). "Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America". Clin Infect Dis. 50 (2): 133–64. doi:10.1086/649554. PMID 20034345.