Sandbox:Cherry
Lab findings

image
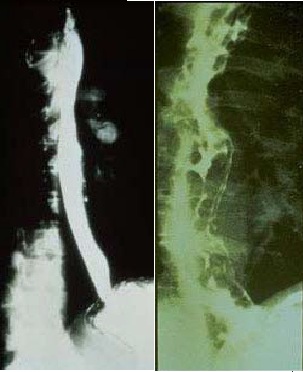
Radiologic findings:
- Radiologic studies include:[1]
- Abdominal ultrasound
- Computed tomography scan
- Magnetic resonance imaging
- Evidence of Portal HTN:
- varices
- Ascites
DIAGNOSIS —
- Abdominal imaging (typically ultrasound) helps:
- Evaluate the liver parenchyma
- Detects extrahepatic manifestations of cirrhosis
Laboratory tests:
- AST to platelet ratio index
- FibroTest/FibroSure
Imaging studies:
- Findings on abdominal imaging are viewed in light of other signs of cirrhosis, such as physical examination or laboratory test findings.
- In addition to evaluating the liver, abdominal imaging may reveal:
- Hepatocellular carcinoma
- Extrahepatic findings suggestive of cirrhosis:
- Ascites
- Varices
- Splenomegaly
- Hepatic or portal vein thrombosis
- Imaging may indicate etiology of cirrhosis:
CT
- Computed tomography (CT) scanning complements ultrasound imaging.
- Classical appearances in some diseases:
- Haemochromatosis: where the excess iron deposition causes a dramatic increase in hepatic density.
- A hypertrophied caudate lobe discovered on computed tomographic (CT) scanning, for example, suggests Budd-Chiari syndrome.
MRI[9][10][11][12][13][14][15]
- Magnetic resonance imaging (MRI):[10][12][16][17]
- determines the nature of focal lesions such as hepatic metastases
- nodular regeneration
- Decreased signal intensity on magnetic resonance imaging may indicate iron overload from hereditary hemochromatosis.
Ultrasonography: routinely used during the evaluation of cirrhosis
- First-line investigation of choice.
- The first radiologic study obtained due to easy availability.
- Provides information about :
- appearance of the liver
- blood flow within the portal circulation
- less expensive than other imaging modalities
- No exposure to intravenous contrast or radiation
- Noninvasive
- cheap
- safe
- well tolerated
- widely available
- Ultrasound, particularly with colour Doppler imaging : [18]
- measures changes in blood flow in the presence of portal hypertension
- excludes biliary obstruction in patients who present with jaundice
- Early signs of cirrhosis in B-ultrasonography include:
- inhomogeneity of the hepatic tissue
- Irregularity of the hepatic surface
- enlargement of the caudate lobe
- Splenomegaly due to portal HTN
- The diagnostic evaluation of cirrhosis with ultrasonography is based on the direct relation between the extent of fibrosis and the ultrasonographically determined degree of liver stiffness.
- ultrasonography can rule cirrhosis in or out in over 90% of cases , its findings are less than 100% specific because of occasional in -correct measurements and false-positive findings
- Findings:[3][19][20][21][22]
- Advanced cirrhosis: liver may appear small and nodular
- Surface nodularity and increased echogenicity with irregular appearing areas are consistent with cirrhosis
- Usually atrophy of the right lobe and hypertrophy of the caudate or left lobes.
- Ultrasonography may also be used as a screening test for hepatocellular carcinoma :nodules on ultrasonography warrants further evaluation
- Findings of portal hypertension:
- increased diameter of the portal vein
- presence of collateral veins
- decreased flow within the portal circulation on Doppler imaging
- Ultrasonography is also useful for detecting splenomegaly, ascites, and portal vein thrombosis.
Computed tomography – not routinely used in the diagnosis of cirrhosis
- It provides similar information to ultrasonography, but at the expense of radiation and contrast exposure.
- CT findings:
- Hepatic nodularity
- Atrophy of the right lobe
- Hypertrophy of the caudate or left lobes
- Ascites
- Varices
- CT portal phase imaging:
- Patency of the portal vein can be demonstrated
Magnetic resonance imaging:
- The role of magnetic resonance imaging (MRI) in the diagnosis of cirrhosis is unclear.
- Use is limited by expense
- Poor tolerance of the examination
- Ability to obtain information provided by MRI through other means
- reveal iron overload and provide an estimate of the hepatic iron concentration
- Magnetic resonance angiography (MRA) is more sensitive than ultrasonography for diagnosing complications of cirrhosis:
- portal vein thrombosis
- CT portal phase imaging, MRA can determine the volume and direction of blood flow in the portal vein.
Elastography: [23][24][25][26][27][28][29][30][31]
- Increasing scarring of the liver is associated with increasing "stiffness" of the tissue.
- Transient elastography and the acoustic radiation force impulse (ARFI) technique are now well-established methods for the staging of fibrosis in various liver diseases [32][33]
Nuclear studies:[34]
- Radionuclide testing can be useful in suggesting the diagnosis of cirrhosis:[35]
- 99mTc sulfur colloid is normally taken up by cells of the reticuloendothelial system
- Cirrhosis: heterogeneity in the uptake of 99mTc sulfur colloid by the liver and increased uptake by the spleen and bone marrow
EGD
EGD[36]
Endoscopic retrograde cholangiopancreatography
- diagnosis of sclerosing cholangitis
Liver biopsy: [37][38][39][40][41][42][43][44]
- Cirrhosis is primarily a histological diagnosis.
- Percutaneous liver biopsy remains the cornerstone of diagnosis.
- quick and simple to perform in a cooperative patient with a normal INR and platelet count.
- The gold standard for diagnosing cirrhosis is:
- Examination of an explanted liver, either at autopsy or following liver transplantation, because the architecture of the entire liver can be appreciated.
- Sample of the liver is obtained by:[47]
- Percutaneous
- Transjugular
- Laparoscopic radiographically-guided fine-needle approach.
- Liver biopsy is not necessary if the clinical, laboratory, and radiologic data strongly suggest the presence of cirrhosis and if the results would not alter the patient's management.
- Patient with a history of heavy alcohol use who has ascites, severe coagulopathy, and a shrunken, nodular-appearing liver on ultrasonography.
- Liver biopsy may be suggestive of etiology:
- Metabolic causes of cirrhosis include:
- Hereditary hemochromatosis
- Nonalcoholic steatohepatitis
- Wilson disease
- Alpha-1 antitrypsin deficiency
- Risks:
- haemorrhage
- biliary peritonitis
- haematoma
- perforation of other viscera
- mortality rates of between 0.01% and 0.1%
- Percutaneous biopsy of focal lesions may be performed in combination with either ultrasound or CT imaging.
- Prerequisites:
- normal INR and platelet count.
- May be performed in combination with either ultrasound or CT.
- Patients with moderate coagulopathy:
- Plugged liver biopsy : injection of gelatin sponges or metal coils down the tract after biopsy
- Laparoscopic liver biopsy performed on a sedated patient with moderate coagulopathy
- Advantage: allows direct visualisation of the liver
- Patients with severe clotting disorders:
- Transjugular liver biopsy :
- risk of intraperitoneal bleed is less
- Disadvantages:
- biopsies are small: multiple biopsies required
- taken 'blindly'
rough
Physical Examination

GIF maker

Source:Wikimedia commons[48]

Source:Wikimedia commons

Source:Wikimedia commons
Physical Examination
- Physical examination of patients with cirrhosis is usually remarkable for: jaundice, spider angiomata, ascites, asterixis, spleenomegaly and palmar erythema.
Appearance of the Patient
- Patients with cirrhosis usually appear weak due to constitutional symptoms such as weight loss, anorexia and muscle atrophy. Yellowish discoloration of skin and abdominal distension may also be present due to ascites.
- Normal/low blood pressure with normal pulse pressure.
Skin
- Jaundice : yellow discoloration of the skin, eyes, and mucus membranes due to increased bilirubin (at least 2-3 mg/dL or 30 mmol/L). Urine may also appear dark.
- Pallor
- Bruises
- Palmar erythema on the thenar and hypothenar eminences, due to altered sex hormone metabolism.
- Spider angiomata: Increased estradiol levels lead to the formation of vascular lesions consisting of central arterioles surrounded by smaller vessels [49]
- Telangiectasias or spider veins: small dilated blood vessels near the surface of the skin.
HEENT
- Abnormalities of the head/hair may include thinning of hair on the scalp due to hyperestrogenism
- Kayser-Fleischer rings : dark rings that appear to encircle the iris of the eye in patients with Wilson's disease.
- Parotid gland enlargement
- Fetor hepaticus: severe portal-systemic shunting leads to increased levels of dimethyl sulfide leads to a sweet pungent smell in the breath
Abdomen
- Inspection:
- Palpation:
- Fluid wave
- Hepatomegaly may be present in initial stages. The liver may also be normal or shrunken.
- Spleenomegaly may be present in patients with cirrhosis from nonalcoholic etiologies, due to portal hypertension
- Percussion:
- Flank dullness may be present due to ascites (needs approximately 1500ml for detection)
- Auscultation:
- Cruveilhier-Baumgarten murmur: venous hum that may be present in patients with portal hypertension.
- Mechanism: due to collateral connections between remnant of the umbilical vein and the portal system
- Location: Epigastrium
- Exacerbating factors: Valsalva maneuver
- Diminished by: application of pressure on the skin above the umbilicus
- Cruveilhier-Baumgarten murmur: venous hum that may be present in patients with portal hypertension.
Genitourinary
- Testicular atrophy
- Inversion of the normal male pubic hair pattern
Neuromuscular
- Hepatic encephalopathy may have signs of:
- Alteration of mental status
- Confusion
- Coma
- Asterixis (bilateral but asynchronous flapping motions of outstretched, dorsiflexed hands) is seen in patients with hepatic encephalopathy.
Extremities
- edema of the lower extremities
- Muscle atrophy
- Nail changes:
- Muehrcke nails: paired horizontal white bands separated by normal color due to hypoalbuminemia
- Terry nails: the proximal two-thirds of the nail plate appears white, whereas the distal one-third is red due to hypoalbuminemia
- Clubbing: the angle between the nail plate and proximal nail fold is greater than 180 degrees
- Severe clubbing:
- "Drum stick" appearance of distal fingers
- Hypertrophic osteoarthropathy: chronic proliferative periostitis of the long bones
- Dupuytren's contracture may cause flexion deformities of the fingers: This occurs due to shortening and thickening of the palmar fascia, due to collagen deposition and fibroblastic proliferation.
- Asterixis in cases with hepatic encephalopathy
Chest findings
- Gynecomastia: due to increased estradiol levels
- Loss of chest or axillary hair
Other findings
History
Psychosocial history
- Past history of abuse
Past Medical history
- History of
Menstrual history
- History of
Family history
- Family history of:
Medication history
- History of medication use should be obtained as many drugs such as opioids cause constipation as a side effect.
Causes
| Drugs and Toxins | Infections | Autoimmune | Metabolic | Biliary obstruction(Secondary bilary cirrhosis) | Vascular | Miscellaneous |
|---|---|---|---|---|---|---|
| Alcohol | Hepatitis B | Primary Biliary Cirrhosis | Wilson's disease | Cystic fibrosis | Chronic RHF | Sarcoidosis |
| Methotrexate | Hepatitis C | Autoimmune hepatitis | Hemochromatosis | Biliary atresia | Budd-Chiari syndrome | Intestinal
bypass operations for obesity |
| Isoniazid | Schistosoma japonicum | Primary Sclerosing Cholangitis | Alpha-1 antitrypsin deficiency | Bile duct strictures | Veno-occlusive disease | Cryptogenic: unknown |
| Methyldopa | Porphyria | Gallstones | ||||
| Glycogen storage diseases (such as Galactosaemia, Abetalipoproteinaemia) |
Cirrhosis
Pathophysiology [50][51][52][53][54][55]
- When an injured issue is replaced by a collagenous scar, it is termed as fibrosis.
- When fibrosis of the liver reaches an advanced stage where distortion of the hepatic vasculature also occurs, it is termed as cirrhosis of the liver.
- The cellular mechanisms responsible for cirrhosis are similar regardless of the type of initial insult and site of injury within the liver lobule.
- Viral hepatitis involves the periportal region, whereas involvement in alcoholic liver disease is largely pericentral.
- If the damage progresses, panlobular cirrhosis may result.
- Cirrhosis involves the following steps: [56]
- Inflammation
- Hepatic stellate cell activation
- Angiogenesis
- Fibrogenesis
- Kupffer cells are hepatic macrophages responsible for Hepatic Stellate cell activation during injury.
- The hepatic stellate cell (also known as the perisinusoidal cell or Ito cell) plays a key role in the pathogenesis of liver fibrosis/cirrhosis.
- Hepatic stellate cells(HSC) are usually located in the subendothelial space of Disse and become activated to a myofibroblast-like phenotype in areas of liver injury.
- Collagen and non collagenous matrix proteins responsible for fibrosis are produced by the activated Hepatic Stellate Cells(HSC).
- Hepatocyte damage causes the release of lipid peroxidases from injured cell membranes leading to necrosis of parenchymal cells.
- Activated HSC produce numerous cytokines and their receptors, such as PDGF and TGF-f31 which are responsible for fibrogenesis.
- The matrix formed due to HSC activation is deposited in the space of Disse and leads to loss of fenestrations of endothelial cells, which is a process called capillarization.
- Cirrhosis leads to hepatic microvascular changes characterised by [57]
- formation of intra hepatic shunts (due to angiogenesis and loss of parenchymal cells)
- hepatic endothelial dysfunction
- The endothelial dysfunction is characterised by [58]
- insufficient release of vasodilators, such as nitric oxide due to oxidative stress
- increased production of vasoconstrictors (mainly adrenergic stimulation and activation of endothelins and RAAS)
- Fibrosis eventually leads to formation of septae that grossly distort the liver architecture which includes both the liver parenchyma and the vasculature. A cirrhotic liver compromises hepatic sinusoidal exchange by shunting arterial and portal blood directly into the central veins (hepatic outflow). Vascularized fibrous septa connect central veins with portal tracts leading to islands of hepatocytes surrounded by fibrous bands without central veins.[59][60][61]
- The formation of fibrotic bands is accompanied by regenerative nodule formation in the hepatic parenchyma.
- Advancement of cirrhosis may lead to parenchymal dysfunction and development of portal hypertension.
- Portal HTN results from the combination of the following:
- Structural disturbances associated with advanced liver disease account for 70% of total hepatic vascular resistance.
- Functional abnormalities such as endothelial dysfunction and increased hepatic vascular tone account for 30% of total hepatic vascular resistance.
Pathogenesis of Cirrhosis due to Alcohol:
- More than 66 percent of all American adults consume alcohol.
- Cirrhosis due to alcohol accounts for approximately forty percent of mortality rates due to cirrhosis.
- Mechanisms of alcohol-induced damage include:
- Impaired protein synthesis, secretion, glycosylation
- Ethanol intake leads to elevated accumulation of intracellular triglycerides by:
- Lipoprotein secretion
- Decreased fatty acid oxidation
- Increased fatty acid uptake
- Alcohol is converted by Alcohol dehydrogenase to acetaldehyde.
- Due to the high reactivity of acetaldehyde, it forms acetaldehyde-protein adducts which cause damage to cells by:
- Trafficking of hepatic proteins
- Interrupting microtubule formation
- Interfering with enzyme activities
- Damage of hepatocytes leads to the formation of reactive oxygen species that activate Kupffer cells.[55]
- Kupffer cell activation leads to the production of profibrogenic cytokines that stimulates stellate cells.
- Stellate cell activation leads to the production of extracellular matrix and collagen.
- Portal triads develop connections with central veins due to connective tissue formation in pericentral and periportal zones, leading to the formation of regenerative nodules.
- Shrinkage of the liver occurs over years due to repeated insults that lead to:
- Loss of hepatocytes
- Increased production and deposition of collagen
Pathology
- There are four stages of Cirrhosis as it progresses:
- Chronic nonsuppurative destructive cholangitis - inflammation and necrosis of portal tracts with lymphocyte infiltration leading to the destruction of the bile ducts.
- Development of biliary stasis and fibrosis
- Periportal fibrosis progresses to bridging fibrosis
- Increased proliferation of smaller bile ductules leading to regenerative nodule formation.
Classification
|
Cirrhosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case studies |
|
Sandbox:Cherry On the Web |
|
American Roentgen Ray Society Images of Sandbox:Cherry |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Charmaine Patel, M.D. [2]
Overview
Cirrhosis of the liver can be classified using two methods; classification based on etiology, and classification based on morphology. Currently, classifying cirrhosis based on morphology is not used, as it requires an invasive procedure to examine the gross appearance of the liver, and it provides little diagnostic value. Classifying cirrhosis according to etiology is a more accepted form of classification, as it can be attained through non-invasive laboratory testing, and has a higher diagnostic value.
Classification Based on Etiology
Cirrhosis can be classified by its etiology. This is the most widely accepted method of classification.
Alcoholic Cirrhosis
This is the most common cause of cirrhosis, and is caused by continuous and prolonged alcohol abuse. The American Academy of Family Physicians estimate that 60-70 percent of all cases of cirrhosis are a result of alcohol abuse.
Post-Necrotic Cirrhosis
This type of cirrhosis occurs after a massive event causes liver cell death. Viral hepatitis is the most common cause for this type of cirrhosis. Agents that are toxic to the liver can also cause this type of cirrhosis, as well as certain types of carcinomas.
Biliary Cirrhosis
This type of cirrhosis results from any diseases that cause biliary obstruction. There is usually a blockage in the bile duct and there may also be inflammation. The excess bile in the liver causes tissue destruction. It commonly results in jaundice.
Cardiac Cirrhosis
This type of cirrhosis is caused by congestive heart failure causing poor circulation of oxygenated blood to the liver. This results in liver cell death, and the subsequent replacement of dead cells by fibrous tissue.
Genetic Disorder
This is when the cirrhosis is caused by a genetic disorder such as hemochromatosis, Wilson's disease, or alpha-1 antitrypsin deficiency.
Malnutrition
This category contains cirrhosis caused by various forms of malnutrition, particularly chronic starvation.
Classification Based on Morphology
Cirrhosis has historically been classified upon the nodular morphology that is seen on upon the gross appearance of the liver. Accurate assessment of the liver morphology can only be obtained through surgery, biopsy, or autopsy, therefore more recently, more non-invasive means of classifying and determining the causes of cirrhosis are used.
| Micronodular | Macronodular | Mixed |
|---|---|---|
| Micronodular cirrhosis is characterized by nodules that are less than 3mm in diameter | Macronodular cirrhosis is characterized by nodules that are more than 3mm in diameter | Micronodular cirrhosis can often progress into macronodular cirrhosis. During this transformation, a mixed form of cirrhosis may be seen.[62] |
Causes:
|
Causes:
|
Mixed nodular cirrhosis is also seen in Indian childhood cirrhosis. [63] |
Video codes
Normal video
{{#ev:youtube|x6e9Pk6inYI}}
Video in table
Floating video
| Title |
| https://https://www.youtube.com/watch?v=ypYI_lmLD7g%7C350}} |
Redirect
- REDIRECTEsophageal web
synonym website
https://mq.b2i.sg/snow-owl/#!terminology/snomed/10743008
Image

Image to the right
 |
Image and text to the right
<figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline>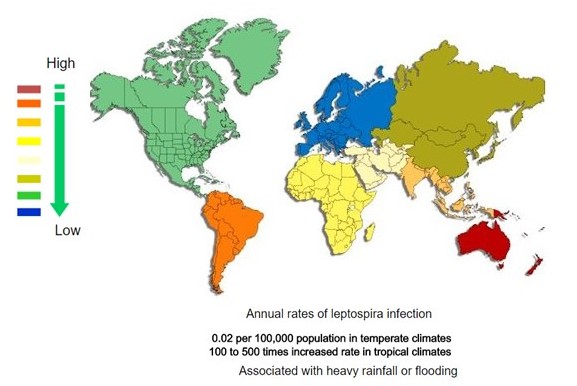 </figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline> Recent out break of leptospirosis is reported in Bronx, New York and found 3 cases in the months January and February, 2017.
</figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline> Recent out break of leptospirosis is reported in Bronx, New York and found 3 cases in the months January and February, 2017.
Gallery
-
Histopathology of a pancreatic endocrine tumor (insulinoma). Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[64]
-
Histopathology of a pancreatic endocrine tumor (insulinoma). Chromogranin A immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[64]
-
Histopathology of a pancreatic endocrine tumor (insulinoma). Insulin immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[64]
References
- ↑ Udell JA, Wang CS, Tinmouth J, FitzGerald JM, Ayas NT, Simel DL, Schulzer M, Mak E, Yoshida EM (2012). "Does this patient with liver disease have cirrhosis?". JAMA. 307 (8): 832–42. doi:10.1001/jama.2012.186. PMID 22357834.
- ↑ Becker CD, Scheidegger J, Marincek B (1986). "Hepatic vein occlusion: morphologic features on computed tomography and ultrasonography". Gastrointest Radiol. 11 (4): 305–11. PMID 3533689.
- ↑ 3.0 3.1 Di Lelio A, Cestari C, Lomazzi A, Beretta L (1989). "Cirrhosis: diagnosis with sonographic study of the liver surface". Radiology. 172 (2): 389–92. doi:10.1148/radiology.172.2.2526349. PMID 2526349.
- ↑ Sanford NL, Walsh P, Matis C, Baddeley H, Powell LW (1985). "Is ultrasonography useful in the assessment of diffuse parenchymal liver disease?". Gastroenterology. 89 (1): 186–91. PMID 3891495.
- ↑ Giorgio A, Amoroso P, Lettieri G, Fico P, de Stefano G, Finelli L, Scala V, Tarantino L, Pierri P, Pesce G (1986). "Cirrhosis: value of caudate to right lobe ratio in diagnosis with US". Radiology. 161 (2): 443–5. doi:10.1148/radiology.161.2.3532188. PMID 3532188.
- ↑ Simonovský V (1999). "The diagnosis of cirrhosis by high resolution ultrasound of the liver surface". Br J Radiol. 72 (853): 29–34. doi:10.1259/bjr.72.853.10341686. PMID 10341686.
- ↑ Trinchet JC, Chaffaut C, Bourcier V, Degos F, Henrion J, Fontaine H, Roulot D, Mallat A, Hillaire S, Cales P, Ollivier I, Vinel JP, Mathurin P, Bronowicki JP, Vilgrain V, N'Kontchou G, Beaugrand M, Chevret S (2011). "Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities". Hepatology. 54 (6): 1987–97. doi:10.1002/hep.24545. PMID 22144108.
- ↑ "EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma". J. Hepatol. 56 (4): 908–43. 2012. doi:10.1016/j.jhep.2011.12.001. PMID 22424438.
- ↑ Ernst O, Sergent G, Bonvarlet P, Canva-Delcambre V, Paris JC, L'Herminé C (1997). "Hepatic iron overload: diagnosis and quantification with MR imaging". AJR Am J Roentgenol. 168 (5): 1205–8. doi:10.2214/ajr.168.5.9129412. PMID 9129412.
- ↑ 10.0 10.1 Bonkovsky HL, Rubin RB, Cable EE, Davidoff A, Rijcken TH, Stark DD (1999). "Hepatic iron concentration: noninvasive estimation by means of MR imaging techniques". Radiology. 212 (1): 227–34. doi:10.1148/radiology.212.1.r99jl35227. PMID 10405746.
- ↑ Gandon Y, Guyader D, Heautot JF, Reda MI, Yaouanq J, Buhé T, Brissot P, Carsin M, Deugnier Y (1994). "Hemochromatosis: diagnosis and quantification of liver iron with gradient-echo MR imaging". Radiology. 193 (2): 533–8. doi:10.1148/radiology.193.2.7972774. PMID 7972774.
- ↑ 12.0 12.1 Ito K, Mitchell DG, Hann HW, Kim Y, Fujita T, Okazaki H, Honjo K, Matsunaga N (1999). "Viral-induced cirrhosis: grading of severity using MR imaging". AJR Am J Roentgenol. 173 (3): 591–6. doi:10.2214/ajr.173.3.10470885. PMID 10470885.
- ↑ Ito K, Mitchell DG, Gabata T, Hussain SM (1999). "Expanded gallbladder fossa: simple MR imaging sign of cirrhosis". Radiology. 211 (3): 723–6. doi:10.1148/radiology.211.3.r99ma31723. PMID 10352597.
- ↑ Ito K, Mitchell DG, Hann HW, Outwater EK, Kim Y, Fujita T, Okazaki H, Honjo K, Matsunaga N (1998). "Progressive viral-induced cirrhosis: serial MR imaging findings and clinical correlation". Radiology. 207 (3): 729–35. doi:10.1148/radiology.207.3.9609897. PMID 9609897.
- ↑ Finn JP, Kane RA, Edelman RR, Jenkins RL, Lewis WD, Muller M, Longmaid HE (1993). "Imaging of the portal venous system in patients with cirrhosis: MR angiography vs duplex Doppler sonography". AJR Am J Roentgenol. 161 (5): 989–94. doi:10.2214/ajr.161.5.8273643. PMID 8273643.
- ↑ Choi D, Kim SH, Lim JH, Cho JM, Lee WJ, Lee SJ, Lim HK (2001). "Detection of hepatocellular carcinoma: combined T2-weighted and dynamic gadolinium-enhanced MRI versus combined CT during arterial portography and CT hepatic arteriography". J Comput Assist Tomogr. 25 (5): 777–85. PMID 11584240.
- ↑ Qayyum A, Goh JS, Kakar S, Yeh BM, Merriman RB, Coakley FV (2005). "Accuracy of liver fat quantification at MR imaging: comparison of out-of-phase gradient-echo and fat-saturated fast spin-echo techniques--initial experience". Radiology. 237 (2): 507–11. doi:10.1148/radiol.2372040539. PMID 16244259.
- ↑ Zwiebel WJ (1995). "Sonographic diagnosis of hepatic vascular disorders". Semin. Ultrasound CT MR. 16 (1): 34–48. PMID 7718281.
- ↑ Martínez-Noguera A, Montserrat E, Torrubia S, Villalba J (2002). "Doppler in hepatic cirrhosis and chronic hepatitis". Semin. Ultrasound CT MR. 23 (1): 19–36. PMID 11866220.
- ↑ Tchelepi H, Ralls PW, Radin R, Grant E (2002). "Sonography of diffuse liver disease". J Ultrasound Med. 21 (9): 1023–32, quiz 1033–4. PMID 12216750.
- ↑ Awaya H, Mitchell DG, Kamishima T, Holland G, Ito K, Matsumoto T (2002). "Cirrhosis: modified caudate-right lobe ratio". Radiology. 224 (3): 769–74. doi:10.1148/radiol.2243011495. PMID 12202712.
- ↑ Albrecht T, Blomley MJ, Cosgrove DO, Taylor-Robinson SD, Jayaram V, Eckersley R, Urbank A, Butler-Barnes J, Patel N (1999). "Non-invasive diagnosis of hepatic cirrhosis by transit-time analysis of an ultrasound contrast agent". Lancet. 353 (9164): 1579–83. doi:10.1016/S0140-6736(98)06373-9. PMID 10334257.
- ↑ Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E (2008). "Performance of transient elastography for the staging of liver fibrosis: a meta-analysis". Gastroenterology. 134 (4): 960–74. doi:10.1053/j.gastro.2008.01.034. PMID 18395077.
- ↑ Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC, Beaugrand M (2005). "Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C". Hepatology. 41 (1): 48–54. doi:10.1002/hep.20506. PMID 15690481.
- ↑ Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, Beaugrand M, Palau R (2003). "Transient elastography: a new noninvasive method for assessment of hepatic fibrosis". Ultrasound Med Biol. 29 (12): 1705–13. PMID 14698338.
- ↑ Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D'Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Gilja OH, Havre RF, Jenssen C, Klauser AS, Ohlinger R, Saftoiu A, Schaefer F, Sporea I, Piscaglia F (2013). "EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology". Ultraschall Med. 34 (2): 169–84. doi:10.1055/s-0033-1335205. PMID 23558397.
- ↑ "EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis". J. Hepatol. 63 (1): 237–64. 2015. doi:10.1016/j.jhep.2015.04.006. PMID 25911335.
- ↑ Castera L, Bedossa P (2011). "How to assess liver fibrosis in chronic hepatitis C: serum markers or transient elastography vs. liver biopsy?". Liver Int. 31 Suppl 1: 13–7. doi:10.1111/j.1478-3231.2010.02380.x. PMID 21205132.
- ↑ Chou R, Wasson N (2013). "Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review". Ann. Intern. Med. 158 (11): 807–20. doi:10.7326/0003-4819-158-11-201306040-00005. PMID 23732714.
- ↑ Khallafi H, Qureshi K (2015). "Imaging Based Methods of Liver Fibrosis Assessment in Viral Hepatitis: A Practical Approach". Interdiscip Perspect Infect Dis. 2015: 809289. doi:10.1155/2015/809289. PMC 4686715. PMID 26779260.
- ↑ Singh S, Fujii LL, Murad MH, Wang Z, Asrani SK, Ehman RL, Kamath PS, Talwalkar JA (2013). "Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis". Clin. Gastroenterol. Hepatol. 11 (12): 1573–84.e1–2, quiz e88–9. doi:10.1016/j.cgh.2013.07.034. PMC 3900882. PMID 23954643.
- ↑ Castera L, Pinzani M (2010). "Biopsy and non-invasive methods for the diagnosis of liver fibrosis: does it take two to tango?". Gut. 59 (7): 861–6. doi:10.1136/gut.2010.214650. PMID 20581229.
- ↑ Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, Takahashi H, Yoneda M, Suda T, Zeuzem S, Herrmann E (2012). "Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis". J. Viral Hepat. 19 (2): e212–9. doi:10.1111/j.1365-2893.2011.01537.x. PMID 22239521.
- ↑ Nishikawa H, Osaki Y (2015). "Liver Cirrhosis: Evaluation, Nutritional Status, and Prognosis". Mediators Inflamm. 2015: 872152. doi:10.1155/2015/872152. PMC 4606163. PMID 26494949.
- ↑ McLaren MI, Fleming JS, Walmsley BH, Ackery DM, Taylor I, Karran SJ (1985). "Dynamic liver scanning in cirrhosis". Br J Surg. 72 (5): 394–6. PMID 3995244.
- ↑ Zardi EM, Di Matteo FM, Pacella CM, Sanyal AJ (2014). "Invasive and non-invasive techniques for detecting portal hypertension and predicting variceal bleeding in cirrhosis: a review". Ann. Med. 46 (1): 8–17. doi:10.3109/07853890.2013.857831. PMC 4904298. PMID 24328372.
- ↑ Williams EJ, Iredale JP (1998). "Liver cirrhosis". Postgrad Med J. 74 (870): 193–202. PMC 2360862. PMID 9683971.
- ↑ Blomley MJ, Lim AK, Harvey CJ, Patel N, Eckersley RJ, Basilico R, Heckemann R, Urbank A, Cosgrove DO, Taylor-Robinson SD (2003). "Liver microbubble transit time compared with histology and Child-Pugh score in diffuse liver disease: a cross sectional study". Gut. 52 (8): 1188–93. PMC 1773750. PMID 12865280.
- ↑ Kim CK, Lim JH, Lee WJ (2001). "Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic liver: accuracy of ultrasonography in transplant patients". J Ultrasound Med. 20 (2): 99–104. PMID 11211142.
- ↑ Abdi W, Millan JC, Mezey E (1979). "Sampling variability on percutaneous liver biopsy". Arch. Intern. Med. 139 (6): 667–9. PMID 443970.
- ↑ Bedossa P, Dargère D, Paradis V (2003). "Sampling variability of liver fibrosis in chronic hepatitis C". Hepatology. 38 (6): 1449–57. doi:10.1016/j.hep.2003.09.022. PMID 14647056.
- ↑ Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER (2002). "Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection". Am. J. Gastroenterol. 97 (10): 2614–8. doi:10.1111/j.1572-0241.2002.06038.x. PMID 12385448.
- ↑ Bravo AA, Sheth SG, Chopra S (2001). "Liver biopsy". N. Engl. J. Med. 344 (7): 495–500. doi:10.1056/NEJM200102153440706. PMID 11172192.
- ↑ Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD (2009). "Liver biopsy". Hepatology. 49 (3): 1017–44. doi:10.1002/hep.22742. PMID 19243014.
- ↑ Tannapfel A, Dienes HP, Lohse AW (2012). "The indications for liver biopsy". Dtsch Arztebl Int. 109 (27–28): 477–83. doi:10.3238/arztebl.2012.0477. PMC 3402072. PMID 22833761.
- ↑ Schirmacher P, Fleig WE, Tannapfel A, Langner C, Dries V, Terracciano L, Denk H, Dienes HP (2004). "[Bioptic diagnosis of chronic hepatitis. Results of an evidence-based consensus conference of the German Society of Pathology, of the German Society for Digestive and Metabolic Diseases and of Compensated Hepatitis (HepNet)]". Pathologe (in German). 25 (5): 337–48. doi:10.1007/s00292-004-0692-7. PMID 15278290.
- ↑ Cholongitas E, Quaglia A, Samonakis D, Senzolo M, Triantos C, Patch D, Leandro G, Dhillon AP, Burroughs AK (2006). "Transjugular liver biopsy: how good is it for accurate histological interpretation?". Gut. 55 (12): 1789–94. doi:10.1136/gut.2005.090415. PMC 1856467. PMID 16636018.
- ↑ "Category:Histopathology of cirrhosis - Wikimedia Commons".
- ↑ Li CP, Lee FY, Hwang SJ; et al. (1999). "Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function". Scand. J. Gastroenterol. 34 (5): 520–3. PMID 10423070.
- ↑ Arthur MJ, Iredale JP (1994). "Hepatic lipocytes, TIMP-1 and liver fibrosis". J R Coll Physicians Lond. 28 (3): 200–8. PMID 7932316.
- ↑ Friedman SL (1993). "Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies". N. Engl. J. Med. 328 (25): 1828–35. doi:10.1056/NEJM199306243282508. PMID 8502273.
- ↑ Iredale JP (1996). "Matrix turnover in fibrogenesis". Hepatogastroenterology. 43 (7): 56–71. PMID 8682489.
- ↑ Gressner AM (1994). "Perisinusoidal lipocytes and fibrogenesis". Gut. 35 (10): 1331–3. PMC 1374996. PMID 7959178.
- ↑ Iredale JP (2007). "Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ". J. Clin. Invest. 117 (3): 539–48. doi:10.1172/JCI30542. PMC 1804370. PMID 17332881.
- ↑ 55.0 55.1 Arthur MJ (2002). "Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C". Gastroenterology. 122 (5): 1525–8. PMID 11984538.
- ↑ Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G (1995). "Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension". Hepatology. 21 (5): 1238–47. PMID 7737629.
- ↑ Fernández M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J (2009). "Angiogenesis in liver disease". J. Hepatol. 50 (3): 604–20. doi:10.1016/j.jhep.2008.12.011. PMID 19157625.
- ↑ García-Pagán JC, Gracia-Sancho J, Bosch J (2012). "Functional aspects on the pathophysiology of portal hypertension in cirrhosis". J. Hepatol. 57 (2): 458–61. doi:10.1016/j.jhep.2012.03.007. PMID 22504334.
- ↑ Schuppan D, Afdhal NH (2008). "Liver cirrhosis". Lancet. 371 (9615): 838–51. doi:10.1016/S0140-6736(08)60383-9. PMC 2271178. PMID 18328931.
- ↑ Desmet VJ, Roskams T (2004). "Cirrhosis reversal: a duel between dogma and myth". J. Hepatol. 40 (5): 860–7. doi:10.1016/j.jhep.2004.03.007. PMID 15094237.
- ↑ Wanless IR, Nakashima E, Sherman M (2000). "Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis". Arch. Pathol. Lab. Med. 124 (11): 1599–607. doi:10.1043/0003-9985(2000)124<1599:ROHC>2.0.CO;2. PMID 11079009.
- ↑ Fauerholdt L, Schlichting P, Christensen E, Poulsen H, Tygstrup N, Juhl E (1983). "Conversion of micronodular cirrhosis into macronodular cirrhosis". Hepatology. 3 (6): 928–31. PMID 6629323.
- ↑ Nayak NC, Ramalingaswami V (1975). "Indian childhood cirrhosis". Clin Gastroenterol. 4 (2): 333–49. PMID 47794.
- ↑ 64.0 64.1 64.2 Neuroendocrine tumor of the pancreas. Libre Pathology. http://librepathology.org/wiki/index.php/Neuroendocrine_tumour_of_the_pancreas
REFERENCES
![Histopathology of a pancreatic endocrine tumor (insulinoma). Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[64]](/images/2/2f/Pancreatic_insulinoma_histology_2.JPG)
![Histopathology of a pancreatic endocrine tumor (insulinoma). Chromogranin A immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[64]](/images/a/a3/Pancreatic_insulinoma_histopathology_3.JPG)
![Histopathology of a pancreatic endocrine tumor (insulinoma). Insulin immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[64]](/images/d/d5/Pancreatic_insulinoma_histology_4.JPG)