Sacrococcygeal teratoma pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| Line 4: | Line 4: | ||
==Overview== | ==Overview== | ||
Sacrococcygeal teratoma originates from the pluripotent cells in primitive knot or Hensen's node, which is the primary organizer of embryonic development, located on the anterior surface of the sacrum or coccyx by 2rd or 3rd gestational week.<ref>http://www.hindawi.com/journals/criog/2012/131369/</ref> Development of Sacrococcygeal teratoma is associated with gain of chromosomes 1q32-qter regions and losses of the 6q24-qter and 18q21-qter regions.<ref name = "aa">{{cite journal |vauthors=Harms D, Zahn S, Göbel U, Schneider DT |title=Pathology and molecular biology of teratomas in childhood and adolescence |journal=Klin Padiatr |volume=218 |issue=6 |pages=296–302 |year=2006 |pmid=17080330 |doi=10.1055/s-2006-942271 |url=}}</ref><ref name = "aaa">{{cite journal |vauthors=Veltman I, Veltman J, Janssen I, Hulsbergen-van de Kaa C, Oosterhuis W, Schneider D, Stoop H, Gillis A, Zahn S, Looijenga L, Göbel U, van Kessel AG |title=Identification of recurrent chromosomal aberrations in germ cell tumors of neonates and infants using genomewide array-based comparative genomic hybridization |journal=Genes Chromosomes Cancer |volume=43 |issue=4 |pages=367–76 |year=2005 |pmid=15880464 |doi=10.1002/gcc.20208 |url=}}</ref> The pathophysiology of sacrococcygeal teratoma depends on the histological subtype. | |||
==Pathogenesis== | ==Pathogenesis== | ||
Sacrococcygeal | Sacrococcygeal teratoma originates from the pluripotent cells in primitive knot or Hensen's node, which is the primary organizer of embryonic development, located on the anterior surface of the sacrum or coccyx by 2rd or 3rd gestational week.<ref>http://www.hindawi.com/journals/criog/2012/131369/</ref> | ||
==Genetics== | ==Genetics== | ||
Development of Sacrococcygeal teratoma is associated with | Development of Sacrococcygeal teratoma is associated with gain of chromosomes 1q32-qter regions and losses of the 6q24-qter and 18q21-qter regions.<ref name = "aa">{{cite journal |vauthors=Harms D, Zahn S, Göbel U, Schneider DT |title=Pathology and molecular biology of teratomas in childhood and adolescence |journal=Klin Padiatr |volume=218 |issue=6 |pages=296–302 |year=2006 |pmid=17080330 |doi=10.1055/s-2006-942271 |url=}}</ref><ref name = "aaa">{{cite journal |vauthors=Veltman I, Veltman J, Janssen I, Hulsbergen-van de Kaa C, Oosterhuis W, Schneider D, Stoop H, Gillis A, Zahn S, Looijenga L, Göbel U, van Kessel AG |title=Identification of recurrent chromosomal aberrations in germ cell tumors of neonates and infants using genomewide array-based comparative genomic hybridization |journal=Genes Chromosomes Cancer |volume=43 |issue=4 |pages=367–76 |year=2005 |pmid=15880464 |doi=10.1002/gcc.20208 |url=}}</ref> | ||
==Associated Conditions== | ==Associated Conditions== | ||
Following conditions are associated with: | Following conditions are associated with sacrococcygeal teratoma: | ||
*[[Myelomeningocoele]]<ref>http://radiopaedia.org/articles/sacrococcygeal-teratoma</ref> | |||
*[[Vertebral anomalies]] | |||
*Bladder outlet obstruction | *Bladder outlet obstruction | ||
*Hydronephrosis | *Hydronephrosis | ||
| Line 25: | Line 30: | ||
==Microscopic Pathology== | ==Microscopic Pathology== | ||
*Sacrococcygeal teratoma can be divided into three types depending on the microscopic pathology | *Sacrococcygeal teratoma can be divided into following three types depending on the microscopic pathology: | ||
:*Mature teratoma:may consist of fully differentiated somatic tissue.<ref name = "micro">{{cite journal |vauthors=Calaminus G, Schneider DT, Bökkerink JP, Gadner H, Harms D, Willers R, Göbel U |title=Prognostic value of tumor size, metastases, extension into bone, and increased tumor marker in children with malignant sacrococcygeal germ cell tumors: a prospective evaluation of 71 patients treated in the German cooperative protocols Maligne Keimzelltumoren (MAKEI) 83/86 and MAKEI 89 |journal=J. Clin. Oncol. |volume=21 |issue=5 |pages=781–6 |year=2003 |pmid=12610174 |doi= |url=}}</ref> | :*Mature teratoma:may consist of fully differentiated somatic tissue.<ref name = "micro">{{cite journal |vauthors=Calaminus G, Schneider DT, Bökkerink JP, Gadner H, Harms D, Willers R, Göbel U |title=Prognostic value of tumor size, metastases, extension into bone, and increased tumor marker in children with malignant sacrococcygeal germ cell tumors: a prospective evaluation of 71 patients treated in the German cooperative protocols Maligne Keimzelltumoren (MAKEI) 83/86 and MAKEI 89 |journal=J. Clin. Oncol. |volume=21 |issue=5 |pages=781–6 |year=2003 |pmid=12610174 |doi= |url=}}</ref> | ||
:*Immature teratoma: may consist of small fraction of incompletely differentiated tissue. | :*Immature teratoma: may consist of small fraction of incompletely differentiated tissue. | ||
:*Malignant teratoma: | :*Malignant teratoma: | ||
Revision as of 16:31, 21 December 2015
|
Sacrococcygeal teratoma Microchapters |
|
Diagnosis |
|---|
| Echocardiography and Ultrasound |
|
Treatment |
|
Case Studies |
|
Sacrococcygeal teratoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Sacrococcygeal teratoma pathophysiology |
|
Risk calculators and risk factors for Sacrococcygeal teratoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Mirdula Sharma, MBBS [2]
Overview
Sacrococcygeal teratoma originates from the pluripotent cells in primitive knot or Hensen's node, which is the primary organizer of embryonic development, located on the anterior surface of the sacrum or coccyx by 2rd or 3rd gestational week.[1] Development of Sacrococcygeal teratoma is associated with gain of chromosomes 1q32-qter regions and losses of the 6q24-qter and 18q21-qter regions.[2][3] The pathophysiology of sacrococcygeal teratoma depends on the histological subtype.
Pathogenesis
Sacrococcygeal teratoma originates from the pluripotent cells in primitive knot or Hensen's node, which is the primary organizer of embryonic development, located on the anterior surface of the sacrum or coccyx by 2rd or 3rd gestational week.[4]
Genetics
Development of Sacrococcygeal teratoma is associated with gain of chromosomes 1q32-qter regions and losses of the 6q24-qter and 18q21-qter regions.[2][3]
Associated Conditions
Following conditions are associated with sacrococcygeal teratoma:
- Myelomeningocoele[5]
- Vertebral anomalies
- Bladder outlet obstruction
- Hydronephrosis
- Rectal stenosis or atresia
- Hydrops fetalis
- Cardiomegaly due to vascular shunting and high output cardiac failure
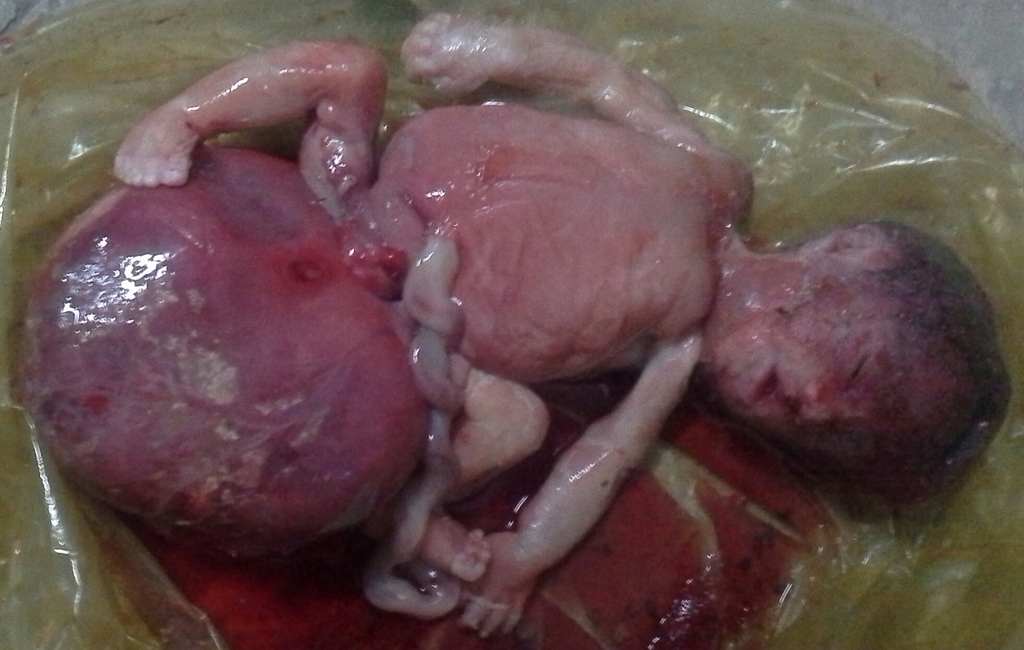
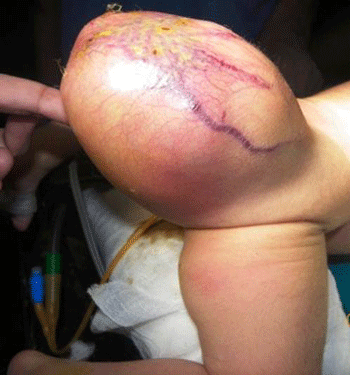
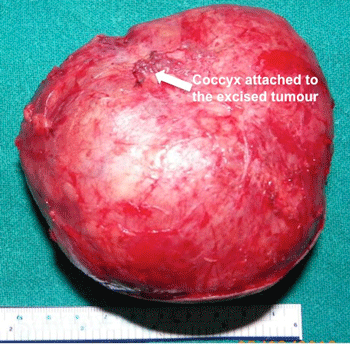
Gross Pathology
-
Gross Image of Sacrococcygeal teratoma
-
Gross Image of Sacrococcygeal teratoma
-
Gross Image of Excised Sacrococcygeal teratoma
Microscopic Pathology
- Sacrococcygeal teratoma can be divided into following three types depending on the microscopic pathology:
- Mature teratoma:may consist of fully differentiated somatic tissue.[6]
- Immature teratoma: may consist of small fraction of incompletely differentiated tissue.
- Malignant teratoma:
- Around 20% of Sacrococcygeal teratomas are malignant.
- They have elevated tumor markers including yolk sac component secreting alpha fetoprotein or primitive neuroectodermal tumor (PNET).
Grading Based Upon Microscopic Features
According to Gonzalez-Crussi System, Sacrococcygeal teratoma is graded on a scale from 0-3, based on the histology:[7][8]
| Grade | Microscopic Features |
|---|---|
|
Grade 0 |
|
|
Grade I |
|
|
Grade II |
|
|
Grade III |
|
References
- ↑ http://www.hindawi.com/journals/criog/2012/131369/
- ↑ 2.0 2.1 Harms D, Zahn S, Göbel U, Schneider DT (2006). "Pathology and molecular biology of teratomas in childhood and adolescence". Klin Padiatr. 218 (6): 296–302. doi:10.1055/s-2006-942271. PMID 17080330.
- ↑ 3.0 3.1 Veltman I, Veltman J, Janssen I, Hulsbergen-van de Kaa C, Oosterhuis W, Schneider D, Stoop H, Gillis A, Zahn S, Looijenga L, Göbel U, van Kessel AG (2005). "Identification of recurrent chromosomal aberrations in germ cell tumors of neonates and infants using genomewide array-based comparative genomic hybridization". Genes Chromosomes Cancer. 43 (4): 367–76. doi:10.1002/gcc.20208. PMID 15880464.
- ↑ http://www.hindawi.com/journals/criog/2012/131369/
- ↑ http://radiopaedia.org/articles/sacrococcygeal-teratoma
- ↑ Calaminus G, Schneider DT, Bökkerink JP, Gadner H, Harms D, Willers R, Göbel U (2003). "Prognostic value of tumor size, metastases, extension into bone, and increased tumor marker in children with malignant sacrococcygeal germ cell tumors: a prospective evaluation of 71 patients treated in the German cooperative protocols Maligne Keimzelltumoren (MAKEI) 83/86 and MAKEI 89". J. Clin. Oncol. 21 (5): 781–6. PMID 12610174.
- ↑ Myers LB, Bulich LA. Anesthesia for Fetal Intervention and Surgery. PMPH-USA; 2005.
- ↑ Harms D, Zahn S, Göbel U, Schneider DT (2006). "Pathology and molecular biology of teratomas in childhood and adolescence". Klin Padiatr. 218 (6): 296–302. doi:10.1055/s-2006-942271. PMID 17080330.