Mammography
|
WikiDoc Resources for Mammography |
|
Articles |
|---|
|
Most recent articles on Mammography Most cited articles on Mammography |
|
Media |
|
Powerpoint slides on Mammography |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Mammography at Clinical Trials.gov Clinical Trials on Mammography at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Mammography
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Mammography Discussion groups on Mammography Patient Handouts on Mammography Directions to Hospitals Treating Mammography Risk calculators and risk factors for Mammography
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Mammography |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Mammography is the process of using low-dose X-rays (usually around 0.7 mSv) to examine the human breast. It is used to look for different types of tumors and cysts. Mammography has been proven to reduce mortality from breast cancer. No other imaging technique has been shown to reduce risk, but self-breast examination (SBE) and physician examination are essential parts of regular breast care. In some countries routine (annual to five-yearly) mammography of older women is encouraged as a screening method to diagnose early breast cancer. Screening mammograms were first proven to save lives in research published by Sam Shapiro, Philip Strax and Louis Venet in 1966.
Like all x-rays, mammograms use doses of ionizing radiation to create this image. Radiologists then analyze the image for any abnormal growths. It is normal to use longer wavelength X-rays (typically Mo-K) than those used for radiography of bones.
At this time, mammography along with physical breast examination is still the modality of choice for screening for early breast cancer. It is the gold-standard which other imaging tests are compared with. CT has no real role in diagnosing breast cancer at the present. Ultrasound, Ductography, and Magnetic Resonance are adjuncts to mammography. Ultrasound is typically used for further evaluation of masses found on mammography or palpable masses not seen on mammograms. Ductograms are useful for evaluation of bloody nipple discharge when the mammogram is non-diagnostic. MRI can be useful for further evaluation of questionable findings, or sometimes for pre-surgical evaluation to look for additional lesions. Stereotactic breast biopsies are another common method for further evaluation of suspicious findings.
Mammography has a false-negative (missed cancer) rate of at least 10 percent. This is partly due to dense tissues obscuring the cancer and the fact that the appearance of cancer on mammograms has a large overlap with the appearance of normal tissues.
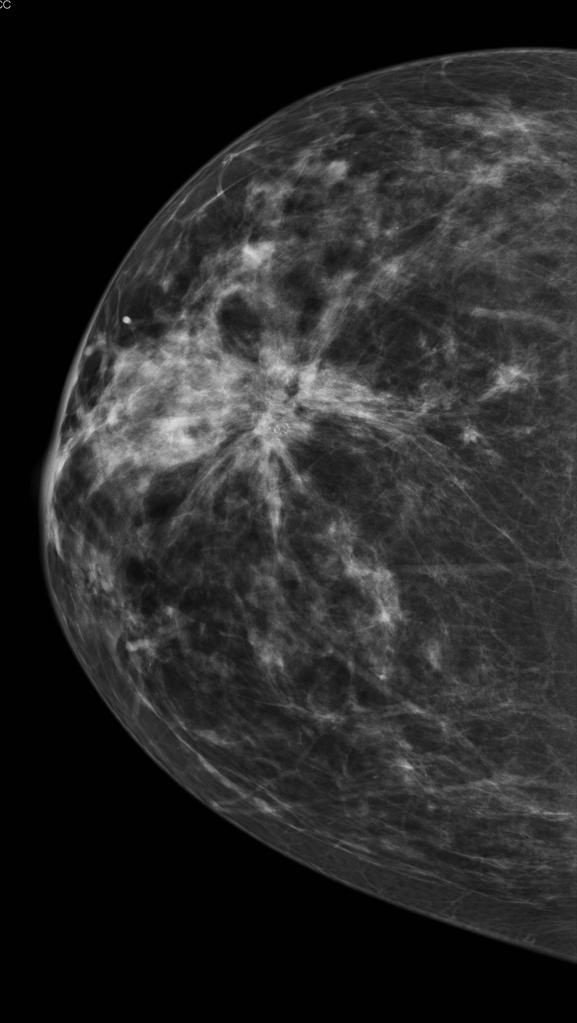
Procedure
During the procedure, the breast is compressed by a dedicated mammography machine to even out the tissue, to increase image quality, and to hold the breast still (preventing motion blur). Both front and side images of the breast are taken. Deodorant, talcum powder or lotion may show up on the X-ray as calcium spots, and women are discouraged from applying these on the day of their investigation.
Until some years ago, mammography was typically performed with screen-film cassettes. Now, mammography is undergoing transition to digital detectors, known as Full Field Digital Mammography (FFDM). This progress is some years later than in general radiology. This is due to several factors:
- the higher resolution demands in mammography,
- significantly increased expense of the equipment,
- the fact that digital mammography has never been shown to be superior to film-screen mammography for the diagnosis of breast cancer.
Computed radiography (CR) may help speed the transition. CR allows facilities to continue to use their existing screen-film units but replace the cassettes with an imaging plate that acts as a digital adapter.
As of March 1, 2007, 18.3% of facilities in the United States and its territories have at least one FFDM unit.[1] (The FDA includes computed radiography units in this figure.[2])
"Work-up" process
In the past several years, the "work-up" process has become quite formalized. It generally consists of screening mammography, diagnostic mammography, and biopsy when necessary. After a screening mammogram, some women may have areas of concern which can't be resolved with only the information available from the screening mammogram. They would then be called back for a "diagnostic mammogram". This phrase essentially means a problem-solving mammogram. During this session, the radiologist will be monitoring each of the additional films as they are taken to determine the cause of the abnormal appearance. Ultrasound is often used at this point, as well.
Generally the cause of the unusual appearance is found to be benign. If the cause cannot be determined to be benign with sufficient certainty, a biopsy will be recommended. The biopsy procedure will be used to obtain actual tissue from the site for the pathologist to examine microscopically to determine the precise cause of the abnormality. In the past, biopsies were most frequently done in surgery, under local or general anesthesia. The majority are now done with needles using either ultrasound or mammographic guidance to be sure that the area of concern is the area that is biopsied.
One study shows that needle biopsies of liver malignancies rarely increase the likelihood that cancer will spread, and has not been found to occur with breast needle biopsies.[2]
Results
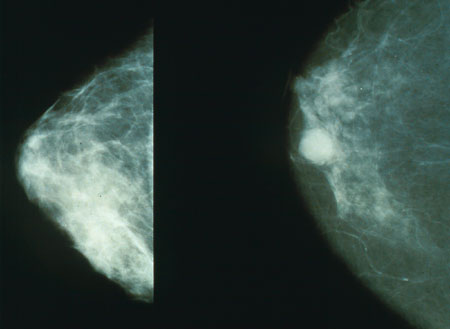
Often women are quite distressed to be called back for a diagnostic mammogram. Most of these recalls will be false positive results. It helps to know these approximate statistics: of every 1,000 U.S. women who are screened, about 7% (70) will be called back for a diagnostic session (although some studies estimate the number closer to 10%-15%). About 10 of these will be referred for a biopsy; the remaining 60 are found to be of benign cause. Of the 10 referred for biopsy, about 3.5 will have a cancer and 6.5 will not. Of the 3.5 who do have cancer, about 2 have a low stage cancer that will be essentially cured after treatment. Mammogram results are often expressed in terms of the BI-RADS Assessment Category, often called a "BI-RADS score." The categories range from 0 (Incomplete) to 6 (Known biopsy – proven malignancy). In the UK mammograms are scored on a scale from 1-5 (1 = normal, 2 = benign, 3 = indeterminate, 4 = suspicious of malignancy, 5 = malignant).
The rates of abnormal and false-positive mammogram results are far lower in countries other than the U.S. that have adopted different quality standards. For example, in Holland, only about 1% of mammograms yield abnormal result. As a result, false-positives are much less common. Despite the higher rates of false-positives in the U.S., women are about as likely to die from breast cancer in the U.S. as in Holland and elsewhere in Europe.
While mammography is the only breast cancer screening method that has been shown to save lives, it is not perfect. Estimates of the numbers of cancers missed by mammography are usually around 10%-20%. This means that of the 350 per 100,000 women who have breast cancer, about 35-70 will not be seen by mammography. Reasons for not seeing the cancer include observer error, but more frequently it is due to the fact that the cancer is hidden by other dense tissue in the breast and even after retrospective review of the mammogram, cannot be seen. Furthermore, one form of breast cancer, lobular cancer, has a growth pattern that produces shadows on the mammogram which are indistinguishable from normal breast tissue.
Computer-aided diagnosis (CAD) are being tested to decrease the number of cases of cancer that are missed in mammograms. In one test, a computer identified 71% of the cases of cancer that had been missed by physicians. However, the computer also flagged twice as many non-cancerous masses than the physicians did. In a second study of a larger set of mammograms, a computer recommended six biopsies that physicians did not. All six turned out to be cancers that would have been missed. (Destounis, et al., 2004) Generally, CAD systems in screening mammography have poor specificity and compare poorly to double reading (Taylor P, Champness J, Given-Wilson R, Johnston K, Potts H (2005). Impact of computer-aided detection prompts on the sensitivity and specificity of screening mammography. Health Technology Assessment, 9(6)).
While data are accumulating suggesting that CAD can find a few additional cancers, this should be put in perspective. The additional find rate was 20%, thus in a group of 1,000 women who will have about 4 cancers, CAD may help find an additional 0.8. The types of additional cancers that may be found are likely to be early and small. As of 2006, there have been no data to show that finding these additional cancers will have any effect on survival rate. Some feel that these cancers are likely to be found at the next screening, still at a curable stage, and therefore it remains to be proven whether CAD will be eventually found to have any effect on patient outcome.
Risks
False positives
The goal of any screening procedure is to examine a large population of patients and find the small number most likely to have a serious condition. These patients are then referred for further, usually more invasive, testing. Thus a screening exam is not intended to be definitive: It is intended to have a high sensitivity so as to not miss any cancers. The cost of this high sensitivity is a relatively large number of results that would be regarded as suspicious in patients without disease. This is true of mammography. The patients called back for further testing from a screening session (about 7%) are sometimes referred to as "false positives", implying an error. In fact, it is essential to call back many healthy patients for further testing to capture as many cases of cancer as possible. These call backs should not be regarded as errors. (See "Results" above.) Nonetheless, some women who receive false-positive results become anxious, worried and distressed about the possibility of having breast cancer, feelings that can last for many years.[3]
False negatives
At the same time, mammograms also have a rate of missed tumors, or "false negatives." Accurate data regarding the number of false negatives are very difficult to obtain, simply because we cannot perform mastectomies on every woman who has had a mammogram to determine the false negative rate accurately. Estimates of the false negative rate depend on close follow-up of a large number of patients for many years. This is difficult in practice, because many women do not return for regular mammography making it impossible to know if they ever developed a cancer. Dr. Samuel S. Epstein, in his book, The Politics of Cancer, claims that in women ages 40 to 49, one in four instances of cancer is missed at each mammography. Researchers have found that breast tissue is denser among younger women, making it difficult to detect tumors. For this reason, false negatives are twice as likely to occur in premenopausal mammograms (Prate.) This is why the screening program in the UK does not start calling women for screening mammograms until the age of 50.
The importance of these missed cancers is not clear, particularly if the woman is getting yearly mammograms. Research on a closely related situation has shown that small cancers that are not acted upon immediately, but are observed over periods of even several years, will have good outcomes. A group of 3,184 women had mammograms which were formally classified as "probably benign." This classification is for patients who are not clearly normal but have some area of minor concern. This results, not in the patient being biopsied, but having early follow up mammography every six months for three years to guarantee no change. Of these 3,184 women, 17 (0.5%) did have cancers. Most importantly, when the diagnosis was finally made, they were all still stage 0 or 1, the earliest stages. Five years after treatment, none of these 17 women had evidence of recurrence. Thus, small early cancers, even though not acted on immediately, were still entirely curable (Sickles, AJR, 179:463-468, 1991).
Regardless of the precise number of false negatives, it is very clear that even if some tumors are missed, lives are saved when they are found. Women need to understand that a negative mammogram is not a perfect guarantee that there is no breast cancer present, but it is the best method we have available.
Other risks
The radiation exposure associated with mammography is a potential risk of screening. The risk of exposure appears to be greater in younger women. The largest study of radiation risk from mammography concluded that for women 40 years of age or older, the risk of radiation-induced breast cancer was minuscule, particularly compared with the potential benefit of mammographic screening, with a benefit-to-risk ratio of 48.5 lives saved for each life lost due to radiation exposure.[4] Organizations such as the National Cancer Institute and United States Preventive Task Force take such risks into account when formulating screening guidelines.[5]
The majority of health experts agree that the risk of breast cancer for women under 35 is not high enough to warrant the risk of radiation exposure. For this reason, and because the radiation sensitivity of the breast in women under 35 is possibly greater than in older women, most radiologists will not perform screening mammography in women under 40. However, if there is a significant risk of cancer in a particular patient (BRCA positive, very positive family history, palpable mass), mammography may still be important. Often, the radiologist will try to avoid mammography, by using ultrasound, or MRI imaging.
Similarly, the risk of breast cancer to women over 55 very clearly justifies the risk of mammograms. The statistics about mammography and women between the ages of 40 and 55 are the most contentious. A 1992 Canadian National Breast Cancer Study showed that mammography had no positive effect on mortality for women between the ages of 40 and 50.[6] This study, however, is the only study to find this result. The study's critics pointed out that there were very serious design flaws in the study that invalidated these results.
While screening between 40 and 50 is still controversial, the preponderance of the evidence indicates that there is some small benefit in terms of early detection. Currently, the American Cancer Society, the National Cancer Institute, and the American College of Radiology encourage mammograms every two years for women ages 40 to 49.[7] In contrast, the American College of Physicians, a large internist group, has recently encouraged individualized screening plans as opposed to wholesale biennual screening of women aged 40 to 49.[8]
Alternatives to mammography
While the cost of mammography is relatively low, its sensitivity is not ideal, with reports listing the range from 45% to about 90% depending on factors such as the density of the breast. Neither is the X-ray based technology completely benign, as noted above. Therefore there is considerable ongoing research into the use of alternative technologies.
One approach, contrast enhanced magnetic resonance imaging (MRI), has shown substantial progress. In this method, the breast is scanned in an MRI device before and after the intravascular injection of a contrast agent (Gadolinium DTPA). The pre-contrast images are "subtracted" from the post-contrast images, and any areas that have increased blood flow are seen as bright spots on a dark background. Since breast cancers generally have an increased blood supply, the contrast agent causes these lesions to "light up" on the images. The available literature suggests that the sensitivity of contrast-enhanced breast MRI is considerably higher than that of either radiographic mammography or ultrasound and is generally reported to be in excess of 95% (though not all reported studies have been as encouraging). The specificity (the confidence that a lesion is cancerous and not a false positive) is only fair, thus a positive finding by MRI should not be interpreted as a definitive diagnosis. The reports of 4,271 breast MRIs from eight large scale clinical trials were reviewed recently by CD Lehman. Overall the sensitivity ranged from 71% to 100% in these reports, however the call-back rates were low at 10% and the risk of having a benign biopsy was reported at 5%, a significant improvement over mammography.
Several medical instrument vendors have entered this arena with breast MRI solutions. One company, Aurora Systems, stands out as being the only manufacturer to make a breast-dedicated unit and as the exclusive patent holder of certain solutions to fat signal suppression that appear to be more or less essential. Siemens, General Electric and Philips Medical, the leading manufacturers of MRI instruments, offer breast MRI products or add-ons, and several third-party companies (e.g., MRI Devices/IGC) offer aftermarket products to enable breast MRI on conventional MRI instruments.
Regulation
Mammography facilities in the United States and its territories (including military bases) are subject to the Mammography Quality Standards Act (MQSA). The act requires annual inspections and accredition every 3 years through an FDA-approved body. Facilities found deficient during the inspection or accreditation process can be barred from performing mammograms until corrective action has been verified or, in extreme cases, can be required to notify past patients that their exams were sub-standard and should not be trusted.
Despite passage of the MQSA by congress in 1992 and the nearly 1 billion dollar cost, the aggregate sensitivity of mammography in the USA is similar to what it was in the 1970s.
At this time MQSA applies only to traditional mammography and not related scans such as breast ultrasound, stereotactic breast biospy, or breast MRI.
References
- ↑ Mammography Quality Scorecard, from the Food and Drug Administration. Updated April 2 2007. Accessed April 9 2007.
- ↑ Mammography Frequently Asked Questions, from the American College of Radiology. Revised January 8 2007; accessed April 9 2007.
- ↑ Brewer NT, Salz T, Lillie SE (2007). "Systematic review: the long-term effects of false-positive mammograms". Ann Intern Med. 146 (7): 502–10. PMID 17404352.
- ↑ Feig S, Hendrick R. "Radiation risk from screening mammography of women aged 40-49 years". J Natl Cancer Inst Monogr: 119–24. PMID 9709287.
- ↑ Screening for Breast Cancer: Recommendations and Rationale. From the United States Preventive Task Force, a section of the Agency for Healthcare Research and Quality. Released February 2002; accessed April 9 2007.
- ↑ Miller AB, Baines CJ, To T, Wall C (1992). "Canadian National Breast Screening Study: 2. Breast cancer detection and death rates among women aged 50 to 59 years". CMAJ. 147 (10): 1477–88. PMC 1336544. PMID 1423088.
- ↑ Screening Mammograms: Questions and Answers, from the National Cancer Institute. Released May 2006; accessed April 9 2007.
- ↑ Screening Mammography for Women 40 to 49 years of age: A Clinical Practice Guideline from the ACP from the American College of Physicians. Released April 2007; accessed April 11 2007.