Huntington's disease
For patient information, click here
| Huntington's disease | |
 | |
|---|---|
| George Huntington's 1872 paper described the disorder. | |
| ICD-10 | G10, F02.2 |
| ICD-9 | 333.4, 294.1 |
| OMIM | 143100 |
| DiseasesDB | 6060 |
| MeSH | D006816 |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Huntington's disease (HD), also mis-spelt as Huntington disease and previously as Huntington's chorea and chorea maior, is a rare inherited neurological disorder affecting up to approximately 10 people per 100,000 people of Western European descent and 0.1 out of 100,000 in people of Asian and African descent. It takes its name from the New York physician George Huntington who described it concisely and precisely in 1872 in his first medical paper. HD has been heavily researched in the last few decades and it was one of the first inherited genetic disorders for which an accurate test could be performed. Huntington's disease is caused by a trinucleotide repeat expansion in the Huntingtin (Htt) gene and is one of several polyglutamine (or PolyQ) diseases. This expansion produces an altered form of the Htt protein, mutant Huntingtin (mHtt), which results in neuronal cell death in select areas of the brain. Huntington's disease is a terminal illness.
Huntington's disease's most obvious symptoms are abnormal body movements called chorea and a lack of coordination, but it also affects a number of mental abilities and some aspects of personality. These physical symptoms occur in a large range of ages,with a mean occurence a person's late forties/early fifties [1]. If the age of onset is below 20 years then it is known as Juvenile HD. As there is currently no proven cure, symptoms are managed with various medications and care methods.
Symptomatology and pathology
Although there is no sudden loss of abilities or exhibition of symptoms, there is a progressive decline. Physical signs are usually the first noticed, but it is unknown how long before the cognitive and psychiatric deficits manifest. Physical symptoms are almost always visible, cognitive symptoms are exhibited differently from person to person, and psychiatric problems may not be evident.
Degeneration of neuronal cells, especially in the frontal lobes, the basal ganglia, and caudate nucleus (the striatum) occurs. There is also astrogliosis and loss of medium spiny neurons. This results in the selective degeneration of the indirect (inhibitory) pathway of the basal ganglia, via the lateral pallidum and the subthalamic nucleus coupled pacemaker system.
Physical
Most people with HD eventually exhibit jerky, random, uncontrollable movements called chorea, although some exhibit very slow movement and stiffness (bradykinesia, dystonia). These abnormal movements are initially exhibited as general lack of coordination and an unsteady gait and gradually increase as the disease progresses; this eventually causes problems with loss of facial expression (called "masks in movement") or exaggerated facial gestures, ability to sit or stand stably, speech, chewing and swallowing (which can lead to weight loss if diet and eating methods are not adjusted accordingly[2][3]), and loss of determination. In the later stages of the disease, speaking is impaired with slurred words and uncontrollable movements of the mouth, eating and mobility are extremely difficult if not impossible, and full-time care is required.
Cognitive
Selective cognitive abilities are progressively impaired, whereas others remain intact. Abilities affected are; executive function (planning; cognitive flexibility, abstract thinking, rule acquisition, initiating appropriate actions, and inhibiting inappropriate actions), psychomotor function (slowing of thought processes to control muscles), speech like slurring of the words and some uncontrollable movement of the lips, perceptual and spatial skills of self and surrounding environment, selection of correct methods of remembering information (but not actual memory itself), and ability to learn new skills, depending on the affected parts of the brain.
Psychopathological
Psychopathological symptoms vary more than cognitive and physical symptoms, and may include anxiety, depression, a reduced display of emotions such as blunting, egocentrism, aggressive behavior, compulsivity which can cause addictions such as alcoholism and gambling, or hypersexuality.
Many patients are unable to recognize expressions of disgust in others and also don't show reactions of disgust to foul odors or tastes.[4] The inability to recognize disgust in others appears in carriers of the Huntington gene before symptoms are manifest.[5] A number of related studies have been published.[6]
Inheritance
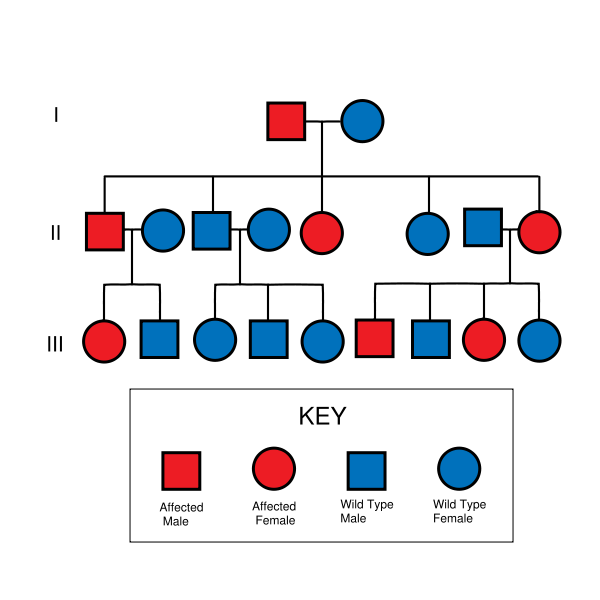
Huntington's disease is autosomal dominant, needing only one affected allele from either parent to inherit the disease. Although this generally means there is a one in two chance of inheriting the disorder from an affected parent, the inheritance of HD and other trinucleotide repeat disorders is more complex.
When the gene has more than 36 copies of the repeated trinucleotide sequence, the DNA replication process becomes unstable and the number of repeats can change in successive generations. If the gene is inherited from the mother, the count is usually similar. Paternal inheritance tends to increase the number of repeats.[7] Because of the progressive increase in length of the repeats, the disease tends to increase in severity and have an earlier onset in successive generations. This is known as anticipation.
De novo mutations are rare.
Homozygous individuals generally do not show an earlier onset of disease, but may have an increased rate of decline.
Causes
The gene involved in Huntington's disease, called the HD gene or Interesting Transcript 15 (IT15), is located on the short arm of chromosome 4 (4p16.3). The end of the HD gene has a sequence of three DNA bases, cytosine-adenine-guanine (CAG), that is repeated multiple times (i.e. ...CAGCAGCAG...); this is called a trinucleotide repeat. CAG is the codon for the amino acid glutamine, thus a CAG repeat may be termed a polyglutamine (polyQ) expansion. A sequence of fewer than 36 glutamine amino acid residues is the normal form, producing a 348 kDa cytoplasmic protein called huntingtin (Htt). A sequence of 40 or more CAG repeats produces a mutated form of Htt, mHtt. The greater the number of CAG repeats, the earlier the onset of symptoms.[8] In genetically altered "knockin" mice, the mutant CAG repeat portion of the gene (which codes for the N-terminal end of mHtt) is all that is needed to cause disease.[9] Aggregates of mHtt are present in the brains of both HD patients[10] and HD mice,[11] specifically in striatal neurons.[12] These aggregates consist mainly of the amino terminal end of mHtt (CAG repeat), and are found in both the cytoplasm and nucleus of neurons.[13] The presence of these aggregates however does not correlate with cell death.[14] Thus mHtt acts in the nucleus but does not cause apoptosis through aggregation.[15]
Mechanism
The exact mechanism by which mHtt causes or contributes towards neuronal cell death and HD symptoms remains unclear. Research exploring the actions of Htt and mHtt have shed light on the subject.
Like all proteins, Htt is translated, performs an action, and is finally degraded. Both Htt and mHtt are cleaved (the first step in degradation) by Caspase-3, which removes the (amino end) N-terminal.[16] Caspase-2 then further breaks down the amino terminal fragment of Htt, but cannot act upon mHtt.[17] The mHtt amino fragments are thus able to affect gene expression in polyQ dependent transcription.[18] Specifically, mHtt binds with TAFII130, a coactivator to CREB dependent transcription.[19] The mHtt N-fragments also interact with SP1, thereby preventing it from binding to DNA.[20] Thus mHtt alters the normal functioning of these proteins. Mutant Huntingtin also downregulates brain-derived neurotropic factor (BDNF) which protects striatal neurons.[21] This loss of BDNF may contribute to striatal cell death, which does not follow apoptotic pathways as the neurons appear to die of starvation.[22] Huntingtin appears to be involved in vesicle trafficking as it interacts with HIT1, a clathrin binding protein, to mediate endocytosis.[23][24]
In the June 16, 2006 issue of Cell, scientists at the University of British Columbia (UBC) and Merck Labs presented findings that the neurodegeneration caused by mHtt is related to the caspase-6 enzyme cleaving the Htt protein. Transgenic mice that have caspase-6 resistant Htt did not show effects of HD.[25] The researchers found "substantial support for the hypothesis that cleavage at the caspase-6 site in mHtt represents a crucial rate-limiting event in the pathogenesis of HD.... Our study highlights the importance of preventing cleavage of Htt at this site and also reinforces the importance of modulating excitotoxicity as a potential therapeutic approach for HD." In essence, scientists have managed to prevent the appearance of HD in genetically modified mice. Dr. Marian DiFiglia, a world-renowned HD researcher and neurobiologist at Harvard University, called this find "very important" and "extremely intriguing".[26]
Diagnosis
To determine whether initial symptoms are evident, a physical and/or psychological examination is required. The uncontrollable movements are often the symptoms which cause initial alarm and lead to diagnosis; however, the disease may begin with cognitive or emotional symptoms, which are not always recognized. Every child of a person with HD has a fifty percent chance of inheriting the faulty copy of the gene and therefore the disease. Pre-symptomatic testing is possible by means of a blood test which counts the number of repetitions in the gene. A negative blood test means that the individual does not carry the expanded copy of the gene, will never develop symptoms, and cannot pass it on to children. A positive blood test means that the individual does carry the expanded copy of the gene, will develop the disease, and has a 50% chance of passing it on to children. A pre-symptomatic positive blood test is not considered a diagnosis, because it may be decades before onset. Because of the ramifications on the life of an at-risk individual, with no cure for the disease and no proven way of slowing it, several counseling sessions are usually required before the blood test. Unless a child shows significant symptoms of the juvenile form or is sexually active or considered to be Gillick competent, children under eighteen will not be tested. The members of the Huntington's Disease Society of America strongly encourage these restrictions in their testing protocol. A pre-symptomatic test is a life-changing event and a very personal decision. For those living in America, there is a list of testing centers available on the HDSA homepage[27] and embryonic genetic screening is also possible, giving mutation-positive or at-risk individuals the option of making sure their children will not inherit the disease. Expense and the ethical considerations of abortion are potential drawbacks to these procedures. The full pathological diagnosis is established by a neurological examination's findings and/or demonstration of cell loss, especially in the caudate nucleus, supported by a cranial CT or MRI scan findings.
Management
There is no treatment to fully arrest the progression of the disease, but symptoms can be reduced or alleviated through the use of medication and care methods. Huntington mice models exposed to better husbandry techniques, better access to water especially, lived much longer than mice who were not well cared for.
Medication
There are treatments available to help control the chorea, although these may have the side effect of aggravating bradykinesia or dystonia.
Other standard treatments to alleviate emotional symptoms include the use of antidepressants and sedatives, with antipsychotics (in low doses) for psychotic symptoms. Care needs to be taken with antipsychotic usage as people suffering psychotic symptoms of organic origin are often more sensitive to the side effects of these drugs.
Nutrition
Nutrition is an important part of treatment; most HD sufferers need two to three times the calories than the average person to maintain body weight, so a nutritionist's advice is needed (the normal population's average daily intake is approximately 2000 calories for women and 2500 for children and men).
Speech therapy can help by improving speech and swallowing methods. This advice should be sought early on, as the ability to learn is reduced as the disease progresses.
To aid swallowing, thickener can be added to drinks. The option of using a stomach PEG is available when eating becomes too hazardous or uncomfortable, this will reduce the chances of pneumonia due to aspiration of food and increase the amount of nutrients and calories that can be ingested.
EPA, an Omega-III fatty acid, slows and possibly reverses the progression of the disease. It is currently in FDA clinical trial, as Miraxion© (LAX-101), for prescription use. Clinical trials utilize 2 grams per day of EPA. In the United States, it is available over the counter in lower concentrations in Omega-III and fish oil supplements.
A calorie restrictive diet delays the onset of symptoms in HD mice.[28]
Potential treatments
Trials and research are conducted on Drosophila fruit flies and mice that have been genetically modified to exhibit HD, before moving on to human trials.
Research is reviewed on various websites for HD sufferers and their families, including the Huntington's Disease Lighthouse, Hereditary Disease Foundation, and Stanford HOPES websites. Primary research can be found by searching the National Library of Medicine's PubMed. Clinical trials of various treatments are ongoing, or yet to be initiated. For example, the US registrar of trials has nine that are currently recruiting volunteers.[29]
Intrabody Therapy
Engineered intracellular antibody fragments (intrabodies) have shown efficacy in vivo as therapeutic agents against pathogenic mutant huntingtin protein in fly models of HD. An intracellularly expressed single-chain Fv against the amino-terminal end of mutant huntingtin (mHtt) has been shown to reduce mHtt aggregate formation and increase turnover of the mHtt fragments in tissue culture models of HD.[30][31] In a drosophila HD model, the expression of this anti-HD intrabody rescued fly survival through the larval and pupal stages to adult emergence. Additionally, the intrabody delayed neurodegeneration in the fly model, and significantly increased the mean adult lifespan.[32] The engineered antibody approach shows promise as a tool for drug discovery and as a potential novel therapeutic for other neurodegenerative disorders resulting from protein misfolding or abnormal protein interactions, including Parkinson’s, Alzheimer’s and prion diseases.[33]
Gene silencing
The most hopeful prospective treatment currently studied is based on interrupting the effects of the HD gene within cells ( gene silencing). Since HD is caused by expression of a single gene, it makes an easier target, and silencing it should halt the progression of the disease. Some success has been achieved with mouse models; in a study with a mouse model of HD treated with siRNA therapy achieved 60% reduction knockdown in expression of the gene and progression of the disease was stalled.[34] ,in another study, mouse models in late stages of the disease recovered their motor functions using doxycycline.[35]
Others
Other agents and measures that have shown promise in initial experiments include dopamine receptor blockers, creatine, CoQ10, the antibiotic Minocycline, exercise, antioxidant-containing foods and nutrients, antidepressants (notably, but not exclusively, selective serotonin reuptake inhibitors SSRIs, such as sertraline, fluoxetine, and paroxetine) and select dopamine antagonists, such as tetrabenazine.
Living Cell Technologies in New Zealand has attempted pig cell implants in trials with positive results in primates, but has yet to conduct a human trial.[36]
Prognosis
Onset of HD seems to be correlated to the number of CAG repeats a person has in their HD gene. Generally, the higher the number of repeats the sooner is the onset.[37] The number of repeats may change slightly with each successive generation, so that the age of onset may vary as well. Symptoms of Huntington’s disease usually become noticeable in the mid 30s to mid 40s.
Juvenile HD has an age of onset anywhere between infancy and 20 years of age. The symptoms of juvenile HD are different from those of adult-onset HD in that they generally progress faster and are more likely to exhibit rigidity and bradykinesia (very slow movement) instead of chorea.
Mortality is due to infection (mostly pneumonia), fall-related injuries, other complications resulting from HD, or suicide (The suicide rate for HD sufferers is much greater than the national average.[38]), rather than the disease itself. Life expectancy is generally between 10 and 25 years after the onset of obvious symptoms.
Epidemiology
The prevalence is 5 to 8 per 100,000, varying geographically.
About 10 percent of HD cases occur in people under the age of 20 years. This is referred to as Juvenile HD, "akinetic-rigid", or "Westphal variant" HD.
Ethical aspects
Whether or not to have the test for HD Genetic counseling may provide perspective for those at risk of the disease. Some choose not to undergo HD testing due to numerous concerns (for example, insurability). Testing of a descendant of a person 'at-risk', has serious ethical implications, as a positive result in a child's test automatically diagnoses the parent.
Parents and grandparents have to decide when and how to tell their children and grandchildren. The issue of disclosure also comes up when siblings are diagnosed with the disease, and especially in the case of identical twins. It is not unusual for entire segments of a family to become alienated as a result of such information or the withholding of it.
For those at risk, or known to have the disease, consideration is necessary prior to having children due to the genetically dominant nature of the disease. In vitro and embryonic genetic screening now make it possible (with 99% certainty) to have an HD-free child; however, the cost of this process can easily reach tens of thousands of dollars. Another consideration regarding genetic testing is the fact that this kind of screening is a form of eugenics. Indeed, historically, Huntington's disease patients were one of the targets groups for the eugenic improvement of the human gene pool. The American scientist Charles Davenport propsed in 1910 that compulsory sterilization and immigration control be aimed at those afflicted with HD (amongst other diseases) [2]
Financial institutions are also faced with the question of whether to use genetic testing results when assessing an individual, e.g. for life insurance. Some countries' organisations have already agreed not to use this information.
History
Research and discovery
- c300 There is evidence that doctors as far back as the Middle Ages may have known of this disease. Along with other conditions with abnormal movements, it may have been referred to as St Vitus' dance. St Vitus is the Christian patron saint of epileptics who was martyred in 303.
- Middle Ages. People with the condition were probably persecuted as being witches or as being possessed by spirits, and were shunned, exiled or worse. Some speculate that the "witches" in the Salem Witch Trials in 1692 had HD.[39]
- 1860 One of the early medical descriptions of HD was made in 1860 by a Norwegian district physician, Johan Christian Lund. He noted that in Setesdalen, a remote and rather secluded area, there was a high prevalence of dementia associated with a pattern of jerking movement disorders that tended to run in families. This is the reason for the disease being commonly referred to as Setesdalsrykkja (Setesdalen=the location, rykkja=jerking movements) in Norwegian.
- 1872 George Huntington was the third generation of a family medical practice in Long Island. With their combined experience of several generations of a family with the same symptoms, he realised their conditions were linked and set about describing it. A year after leaving medical school, in 1872, he presented his accurate definition of the disease to a medical society in Middleport, Ohio.
- c1923 Smith Ely Jelliffe (1866-1945) and Frederick Tilney (1875-1938) began analyzing the history of HD sufferers in New England.
- 1932 P. R. Vessie expanded Jelliffe and Tilney's work, tracing about a thousand people with HD back to two brothers and their families who left Bures in Essex for Suffolk bound for Boston in 1630.
- 1979 The U.S-Venezuela Huntington's Disease Collaborative Research Project began an extensive study which gave the basis for the gene to be discovered. This was conducted in the small and isolated Venezuelan fishing villages of Barranquitas and Lagunetas. Families there have a high presence of the disease, which has proved invaluable in the research of the disease.
- 1983 James Gusella, David Housman, P. Michael Conneally, Nancy Wexler, and their colleagues find the general location of the gene, using DNA marking methods for the first time - an important first step toward the Human Genome Project.
- 1992 Anita Harding,et al. find that trinucleotide repeats affect disease severity[40]
- 1993 The Huntington's Disease Collaborative Research Group isolates the precise gene at 4p16.3.
- 1996 A transgenic mouse ([the R6 line]) was created that could be made to exhibit HD greatly advancing how much experimentation can be achieved.
- 1997 Researchers discovered that mHtt aggregates (misfolds) to form nuclear inclusions.
- "Mayo Clinic Discovers DNA Repair as Key to Huntington's Disease". Mayo Clinic. April 22, 2007. Retrieved 2007-04-23.
- The full record of research is extensive.[41][42][43]
Organizations
- 1967 Woody Guthrie's wife, Marjorie Guthrie, helped found the Committee to Combat Huntington's Disease, after his death whilst suffering from HD. This eventually became the Huntington's Disease Society of America.[44] Since then, lay organizations have been formed in many countries around the world.
- 1968 After experiencing HD in his wife's family Dr. Milton Wexler was inspired to start the Hereditary Disease Foundation (HDF). Professor Nancy S. Wexler, Dr. Wexler's daughter, was in the research team in Venezeula and is now president of the HDF.
- 1974 the first international meeting took place when the founders of the Canadian HD Society (Ralph Walker) and of the British HD Society (Mauveen Jones) attended the annual meeting of the American HD Society
- 1977 second meeting organized by the Dutch Huntington Society the "Vereniging van Huntington", representatives of six countries were present.
- 1979 International Huntington Association (IHA) formed during international meeting in Oxford (England) organized by HDA of England.
- 1981-2001 Biennial meetings held by IHA which became the World Congress on HD.
- 2003 the first World Congress on Huntington's Disease was held in Toronto. This is a biennial meeting for associations and researchers to share ideas and research, which is held on odd-number years. The Euro-HD Network[45] was started as part of the Huntington Project,[46] funded by the High-Q Foundation.[47]
References
- ↑ Adams P, Falek A, Arnold J (1988). "Huntington disease in Georgia: age at onset". Am. J. Hum. Genet. 43 (5): 695–704. PMID 2973230.
- ↑ Gaba AM, Zhang K, Marder K, Moskowitz CB, Werner P, and Boozer CN. Energy balance in early-stage Huntington disease. Am J Clin Nutr. 2005; 81(6):1335-41. PMID 15941884
- ↑ Caregiver's Handbook for Advanced-Stage Huntington Disease. Booklet by the Huntington Society of Canada, retrieved 2007-04-11.
- ↑ Mitchell IJ, Heims H, Neville EA, Rickards H. Huntington's disease patients show impaired perception of disgust in the gustatory and olfactory modalities. Journal of Neuropsychiatry and Clinical Neuroscience, 17:119-121, February 2005. PMID 15746492
- ↑ Sprengelmeyer R, Schroeder U, Young AW, Epplen JT. "Disgust in pre-clinical Huntington's disease: a longitudinal study." Neuropsychologia. 2006;44(4):518-33. Epub 2005 August 11. PMID 16098998
- ↑ PubMed search for "Huntington's disease" and "disgust"
- ↑ RM Ridley, CD Frith, TJ Crow and PM Conneally (1988). "Anticipation in Huntington's disease is inherited through the male line but may originate in the female". Journal of Medical Genetics. 25: 589–595.
- ↑ Kieburtz K, MacDonald M, Shih C, Feigin A, Steinberg K, Bordwell K, Zimmerman C, Srinidhi J, Sotack J, Gusella J, et al. Trinucleotide Repeat Length and Progression of Illness in Huntington's Disease. J Med Genet. 1994; 31:872
- ↑ Murphy K, Carter R, Lione L, Mangiarini L, Mahal A, Bates G, Dunnett S, and Morton J. Abnormal Synaptic Plasticity and Impaired Spatiail Cognition in Mice Transgenic for Exon 1 of the Human Huntington’s Disease Mutation. Journal of Neuroscience 2000; 20:5115
- ↑ Difiglia M, Sapp E, Chase E, Davies K. et al. Aggregation of Huntingtin in Neuronal Intranuclear Inclusions and Dystrophic Neurites in Brain. Science 1997
- ↑ Davies S, Turmaine M, Cozens B, Difiglia M, Sharp A, Ross C, Scherzinger E, Wanker E, Mangiarini L, and Bates G. Formation of Neuronal Intranuclear Inclusions Underlies the Neurological Dysfunction in Mice Transgenic for the HD Mutation. Cell 1997; 90:537
- ↑ Li H, Li S, Johnston H, Shelbourne P, and Li X. Amino-terminal Fragments of Mutant Huntingtin Show Selective Accumulation in Striatal Neurons and Synaptic Toxicity. Nature Genetics 2000; 25:385
- ↑ Cooper JK, Schilling G, Peters MF, Herring WJ, Sharp AH, Kaminsky Z, Masone J, Khan FA, Delanoy M, Borchelt DR, Dawson VL, Dawson TM, Ross CA. Truncated N-terminal Fragments of Huntingtin with Expanded Glutamine Repeats Form Nuclear and Cytoplasmic Aggregates in Cell Culture. Hum Mol Genet. 1998; 7:783
- ↑ F. R. Fusco, Q. Chen, W. J. Lamoreaux, G. Figueredo-Cardenas, Y. Jiao, J. A. Coffman, D. J. Surmeier, M. G. Honig, L. R. Carlock, and A. Reiner. Cellular Localization of Huntingtin in Striatal and Cortical Neurons in Rats: Lack of Correlation with Neuronal Vulnerability in Huntington's Disease. J. Neurosci 1999; 19: 1189
- ↑ Saudou F, Finkbeiner S, Devys, and Greenberg M. Huntingtin Acts in the Nucleus to Induce Apoptosis but Death Does Not Correlate with the Formation of Intranuclear Inclusions. Cell. 1998; 95:55
- ↑ Kim YJ, Yi Y, Sapp E, Wang Y, Cuiffo B, Kegel KB, Qin ZH, Aronin N, DiFiglia M. Caspase 3-cleaved N-terminal Fragments of Wild-type and Mutant Huntingtin are Present in Normal and Huntington's Disease Brains, Associate with Membranes, and Undergo calpain-dependent proteolysis. Proc Natl Acad Sci U S A. 2001; 98:12784
- ↑ Hermel E, Gafni J, Propp SS, Leavitt BR, Wellington CL, Young JE, Hackam AS, Logvinova AV, Peel AL, Chen SF, Hook V, Singaraja R, Krajewski S, Goldsmith PC, Ellerby HM, Hayden MR, Bredesen DE, Ellerby LM. Specific Caspase Interactions and Amplification are Involved in Selective Neuronal Vulnerability in Huntington's Disease. Cell Death Differ 2004; 11:424
- ↑ Freiman R, and Tjian R. A Glutamine-Rich Trail Leads to Transcription Factors. Science 2002; 296:2149
- ↑ Bae B.I, Xu H, Igarashi S, Fujimuro M, Agrawal N, Taya Y, Hayward S.D, Moran T.H, Montell C, Ross C.A, Snyder S.H, and Sawa A. p53 Mediates Cellular Dysfunction and Behavioral Abnormalities in Huntington's Disease. Neuron 2005; 47:29
- ↑ Dunah A, Jeong H, Griffin A, Kim Y, Standeart D, Hersch S, Mouradian M, Young A, Tanese N, and Krainc D. Sp1 and TAFII130 Transcriptional Activity Disrupted in Early Huntington’s Disease. Science 2002; 296: 2238
- ↑ Canals J, Pineda J, Torres-Peraza J, Bosch M, Martin-Ibanez R, Munoz M, Mengod G, Ernfors P, and Alberch J. Brain Derived Neurotrophic Factor Regulates the Onset and Severity of Motor Dysfunction Associated with Enkephalinergic Neuronal Degeneration in Huntington’s Disease. Neurobiology of Disease 2004; 24:7727
- ↑ Sawa A, Nagata E, Sutcliffe S, Dulloor P, Cascio MB, Ozeki Y, Roy S, Ross CA, Snyder SH. Huntingtin is Cleaved by Caspases in the Cytoplasm and Translocated to the Nucleus via Perinuclear Sites in Huntington's Disease Patient Lymphoblasts. Neurobiol Dis 2005
- ↑ Velier J, Kim M, Schwarz C, Kim T.W, Sapp E, Chase K, Aronin N, DiFiglia M. Wild-Type and Mutant Huntingtins Function in Vesicle Trafficking in the Secretory and Endocytic Pathways. Experimental Neurology 1998; 152:34
- ↑ Waelter S, Scherzinger E, Hasenbank R, Nordhoff E, Lurz R, Goehler H, Gauss C, Sathasivam K, Bates G, Lehrach H, and Wanker E. The Huntingtin Interacting Protein HIP1 is a Clathrin and α–adaptin-binding Protein Involved in Receptor Mediated Endocytosis. Human Molecular Genetics 2001; 10:1807
- ↑ Graham, RK, Y Deng, EJ Slow, B Haigh, N Bissada, G Lu, J Pearson, J Shehadeh, L Bertram, Z Murphy, SC Warby, CN Doty, S Roy, CL Wellington, BR Leavitt, LA Raymond, DW Nicholson, MR Hayden (2006-06-16). "Cleavage at the Caspase-6 site is required for neuronal dysfunction and degeneration due to mutant Huntingtin". Cell. 125: 1179–1191.
- ↑ S. Ubelacker. "Canadian Researchers cure Huntington's disease in mice". Retrieved 2006-07-16.
- ↑ www.hdsa.org
- ↑ Fasting Forestalls Huntington's Disease in Mice on friendsoffreedom.org
- ↑ clinicaltrials.gov
- ↑ Lecerf, J. M., Shirley, T. L., Zhu, Q., Kazantsev, A., Amersdorfer, P., Housman, D. E., Messer, A. & Huston, J. S. Human single-chain Fv intrabodies counteract in situ huntingtin aggregation in cellular models of Huntington's disease. (2001) Proc. Natl. Acad. Sci. USA 98, 4764-4769.
- ↑ Miller, T. W., Zhou, C., Gines, S., MacDonald, M. E., Mazarakis, N. D., Bates, G. P., Huston, J. S. & Messer, A. A human single-chain Fv intrabody preferentially targets amino-terminal huntingtin fragments in striatal models of Huntington's disease (2005) Neurobiol. Dis. 19, 47-56.
- ↑ Wolfgang WJ, Miller TW, Webster JM, Huston JS, Thompson LM, Marsh JL, Messer A. Suppression of Huntington's disease pathology in Drosophila by human single-chain Fv antibodies. (2005) Proc Natl Acad Sci USA 32:11563-8.
- ↑ Miller, T. W., Messer, A. Intrabody Applications in Neurological Disorders: Progress and Future Prospects. (2005) Molecular Therapy. 12, 394-401.
- ↑ Harper SQ, Staber PD, He X; et al. (2005). "RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model". Proc. Natl. Acad. Sci. U.S.A. 102 (16): 5820–5. doi:10.1073/pnas.0501507102. PMID 15811941.
- ↑ Miguel Díaz-Hernández, Jesús Torres-Peraza, Alejandro Salvatori-Abarca, María A. Morán, Pilar Gómez-Ramos, Jordi Alberch, and José J. Lucas (October 19, 2005). "Full Motor Recovery Despite Striatal Neuron Loss and Formation of Irreversible Amyloid-Like Inclusions in a Conditional Mouse Model of Huntington's Disease". The Journal of Neuroscience. 25 (42): 9773–9781. Retrieved 2006-07-16.
- ↑ World health Article
- ↑ The Huntington Disease lighthouse.org
- ↑ http://www.huntington-assoc.com/Critical%20ab05.pdf]
- ↑ The brief history of HD on stanford.edu
- ↑ PMID 1303283
- ↑ Achievements of Hereditary Disease Foundation
- ↑ HDA research news - medical research into treatment & prevention on hda.org.uk
- ↑ Bates G, Harper PS, Jones L (2002) Huntington's disease, 3rd Edition. Oxford: Oxford University Press.
- ↑ Huntington's Disease Society of America
- ↑ Euro-HD Network
- ↑ Huntington Project
- ↑ High-Q Foundation
Bibliography
- John P. Conomy, M.D., J.D. "Dr. George Sumner Huntington and the Disease Bearing His Name".
- P. R. Vessie (1932). "On the transmission of Huntington's chorea for 300 years – the Bures family group". Journal of Nervous and Mental Disease, Baltimore. 76: 553–573.
- G. Huntington (1872-04-13). "On Chorea". Medical and Surgical Reporter of Philadelphia. 26 (15): 317–321.
- Gillian Bates, Peter Harper, Lesley Jones (2002). Huntington's Disease - Third Edition. Oxford: Oxford University Press. ISBN 0-19-851060-8.
- Article 143100 - Huntington Disease, Online Mendelian Inheritance in Man, Johns Hopkins University
- Article 606438 - Huntingtons Disease-Like 2 Online Mendelian Inheritance in Man, Johns Hopkins University
- History of Medicine Bulletin,The Johns Hopkins University, a biography of George Huntington and other relevant information.
See also
Template:Diseases of the nervous system Template:SIB
ca:Malaltia de Huntington de:Chorea Huntington fa:بیماری هانتینگتون ko:헌팅턴 무도병 it:Morbo di Huntington he:מחלת הנטינגטון nl:Ziekte van Huntington no:Huntingtons sykdom simple:Huntington's disease sr:Хантингтонова хореа fi:Huntingtonin tauti sv:Huntingtons sjukdom