Minocycline
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Minocycline is a tetracycline antiobiotic that is FDA approved for the treatment of is indicated as an adjunct to scaling and root planing procedures for reduction of pocket depth in patients with adult periodontitis. Minocycline may be used as part of a periodontal maintenance program which includes good oral hygiene, and scaling and root planing. Common adverse reactions include the most frequently reported nondental treatment-emergent adverse events in the 3 multicenter US trials were headache, infection, flu syndrome, and pain. Dental conditions include peritonitis, gingivitis, stomatitis, pharyngitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Minocycline is indicated as an adjunct to scaling and root planing procedures for reduction of pocket depth in patients with adult periodontitis. Minocycline may be used as part of a periodontal maintenance program which includes good oral hygiene, and scaling and root planing.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Minocycline in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Minocycline in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Minocycline FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Minocycline in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Minocycline in pediatric patients.
Contraindications
It should not be used in any patient who has a known sensitivity to tetracyclines.
Warnings
There is limited information regarding Minocycline Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
- This medication may cause upset stomach, diarrhea, dizziness, unsteadiness, drowsiness, headache or vomiting. If these symptoms persist or worsen, one should notify their doctor. Minocycline increases sensitivity to sunlight. Prolonged sun exposure should be avoided. Wear protective clothing and use a sunscreen if needed. Very unlikely but should be reported: fever, yellowing of the eyes or skin, stomach pain, sore throat, vision changes and mental changes.
- In those cases where this drug must be used for extended periods, blue-gray skin discoloration may occur. In the unlikely event one has an allergic reaction to this drug, immediate medical attention should be sought. Symptoms of an allergic reaction include rash, itching, swelling, severe dizziness, trouble breathing. Other effects not listed above should be reported to the doctor or pharmacist.
Postmarketing Experience
There is limited information regarding Minocycline Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Minocycline Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Minocycline in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Minocycline in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Minocycline during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Minocycline in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Minocycline in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Minocycline in geriatric settings.
Gender
There is no FDA guidance on the use of Minocycline with respect to specific gender populations.
Race
There is no FDA guidance on the use of Minocycline with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Minocycline in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Minocycline in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Minocycline in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Minocycline in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Minocycline Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Minocycline and IV administrations.
Overdosage
There is limited information regarding Minocycline overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
There is limited information regarding Minocycline Mechanism of Action in the drug label.
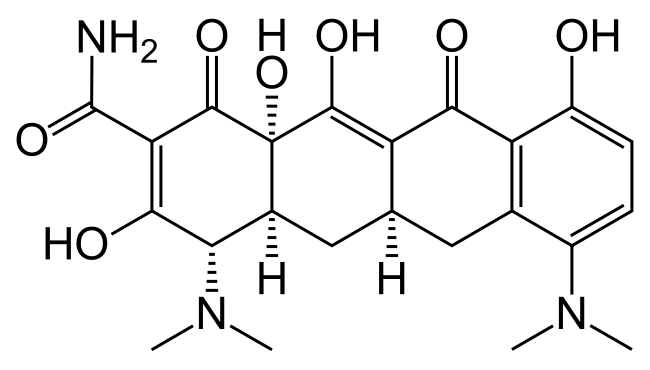
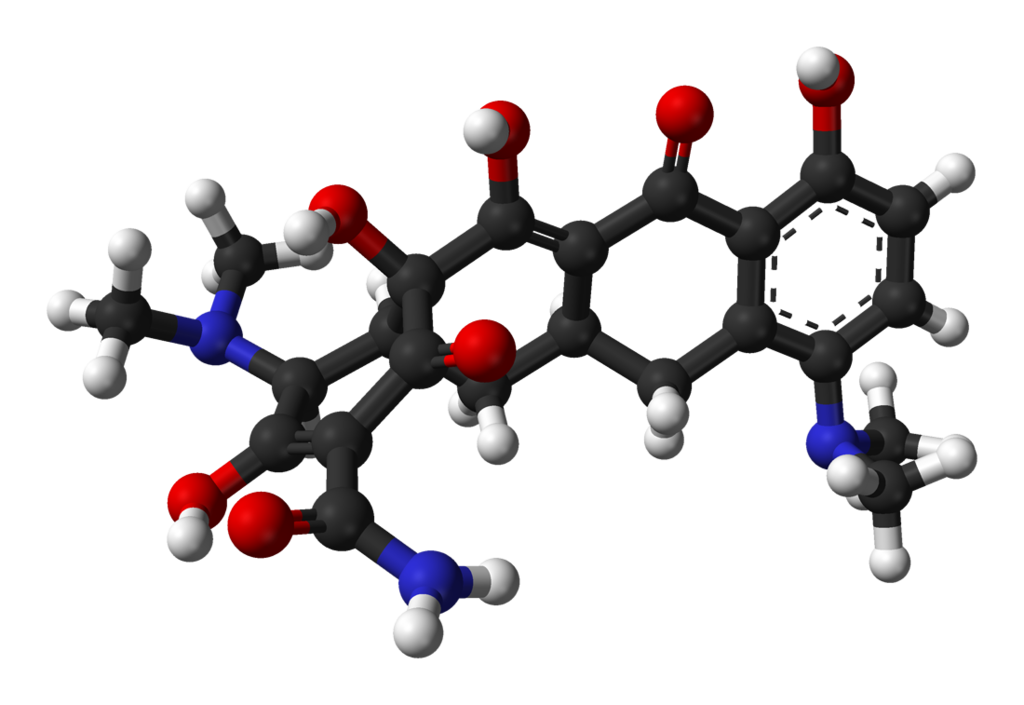
Structure
There is limited information regarding Minocycline Structure in the drug label.
Pharmacodynamics
There is limited information regarding Minocycline Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Minocycline Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Minocycline Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Minocycline Clinical Studies in the drug label.
How Supplied
- Minocycline equivalent to 50 mg minocycline are opaque white capsules imprinted "0487" and "DYNACIN® 50 mg" and are supplied as follows:
NDC 99207-487-10 Bottles of 100 NDC 99207-487-11 Bottle of 1000.
- Minocycline equivalent to 75 mg minocycline are light gray opaque capsules imprinted "0489" and "DYNACIN® 75 mg" and are supplied as follows:
NDC 99207-489-10 Bottles of 100 NDC 99207-489-11 Bottle of 1000.
- Minocycline equivalent to 100 mg minocycline are opaque dark gray and opaque white capsules imprinted "0488" and "DYNACIN® 100 mg" and are supplied as follows:
NDC 99207-488-05 Bottles of 50 NDC 99207-488-11 Bottle of 1000.
- Dispense in tight, light-resistant container with child-resistant closure.
- Store at 20º–25ºC (68º–77ºF).
- Protect from light, moisture and excessive heat.
Storage
There is limited information regarding Minocycline Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Minocycline |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
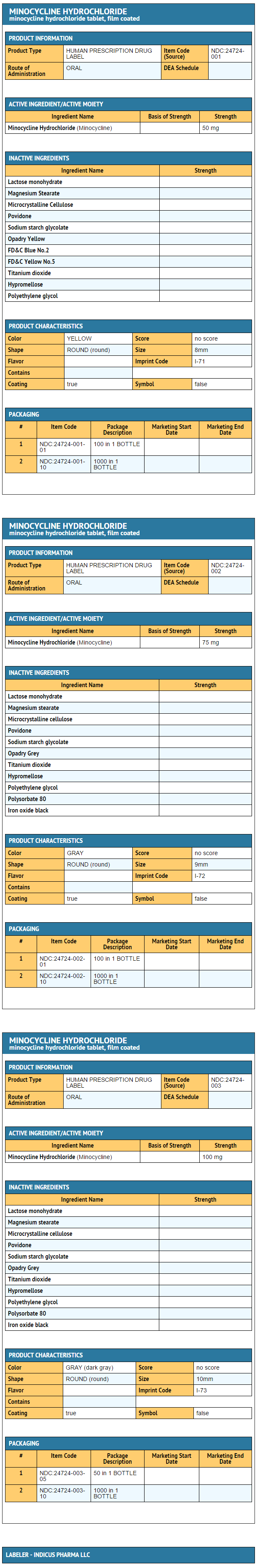
{{#ask: Label Page::Minocycline |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Minocycline Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Minocycline interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- MINOCYCLINE HYDROCHLORIDE ®[2]
Look-Alike Drug Names
There is limited information regarding Minocycline Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.