Hepatitis A
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [5]
{{#meta: itemprop="medicalWebPageAudiences" content="patient"}}{{#meta: itemprop="medicalWebPageSpecialities" content="cardiology"}}{{#meta: itemprop="medicalWebPageInfoTypes" content="symptoms,diagnosis,treatment,causes,prognosis,complications"}} Classification Classic::Classification Atypical::
Overview
|
Hepatitis A |
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Hepatitis A On the Web |
|
American Roentgen Ray Society Images of Hepatitis A |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [6]
Overview
Hepatitis A (formerly known as infectious hepatitis and epidemical virus) is an acute infectious disease of the liver caused by the hepatitis A virus (Hep A),[1] an RNA virus, usually spread the fecal-oral route; transmitted person-to-person by ingestion of contaminated food or water or through direct contact with an infectious person. Tens of millions of individuals worldwide are estimated to become infected with Hep A each year.[2] The time between infection and the appearance of the symptoms (the incubation period) is between two and six weeks and the average incubation period is 28 days.[3] In developing countries, and in regions with poor hygiene standards, the incidence of infection with this virus is high[4] and the illness is usually contracted in early childhood. As incomes rise and access to clean water increases, the incidence of HAV decreases.[5] Hepatitis A infection causes no clinical signs and symptoms in over 90% of infected children and since the infection confers lifelong immunity, the disease is of no special significance to those infected early in life. In Europe, the United States and other industrialized countries, on the other hand, the infection is contracted primarily by susceptible young adults, most of whom are infected with the virus during trips to countries with a high incidence of the disease[3] or through contact with infectious persons.
Historical Perspective
Hepatitis A virus was first identified in 1973. It was classified as a separate disease from other types of hepatitis during World War II. However, its true prevalence and route of transmission would only be recognized later.[6] During 1995-1996, the Food and Drug Administration (FDA) approved the inactivated hepatitis A vaccines. Consequently, hepatitis A became a disease that was not only common but also vaccine-preventable.
Pathophysiology
Hepatitis A is a liver disease caused by the hepatitis A virus (HAV). HAV has fecal-oral transmission and its infectivity peaks about 2-weeks before the onset of jaundice. Gross observation of the liver may show an enlarged and erythematous liver, while microscopically it may reveal lymphocyte infiltration and inflammation of the portal tracts.
Causes
Hepatitis A is a liver disease caused by the hepatitis A virus (HAV). HAV has fecal-oral transmission and its infectivity peaks about 2-weeks before the onset of jaundice. Gross observation of the liver may show an enlarged and erythematous liver, while microscopically it may reveal lymphocyte infiltration and inflammation of the portal tracts.
Differential Diagnosis
Hepatitis A must be differentiated from other diseases that cause fever, nausea, vomiting, jaundice, hepatomegaly, icteric sclera, elevated ALT, AST, and PCR such as other viral hepatitis, alcoholic hepatitis, and autoimmune hepatitis.
Epidemiology and Demographics
The incidence of hepatitis A varies among eras, countries and even cities within the same country. In recent years it has been noted a shift in prevalence, what was once a disease more prevalent in children, is today predominant in adults. In the United States, the incidence of hepatitis A in 2011 was 0.4 cases per 100,000 population. In recent years, the rates of hepatitis A have been similar among all age groups. After the introduction of the HAV vaccine, historic differences in rates of hepatitis A among racial/ethnic populations have also narrowed. In developed countries, elimination of historic geographic differences in incidence rates has also occurred. In developing countries with very poor sanitary conditions and hygienic practices, most children (90%) are infected with the hepatitis A virus before the age of 10.
Risk Factors
Subjects who are not immunized against hepatitis A virus (HAV) and who travel to endemic areas where there is poor sanitation and lack of safe water are at increased risk of contracting HAV infection. Additional risk factors for HAV include intravenous drug injection, living in a household with an infected person, having a sexual partner with acute HAV infection, and attending childcare centers.
Screening
The detection of hepatitis A virus (HAV) antibodies in the blood is used to screen for hepatitis A. Anti-HAV IgG remains elevated after acute disease.[7]
Natural History, Complications and Prognosis
Hepatitis A is caused by infection with hepatitis A virus (HAV) which has an incubation period of approximately 28 days. HAV infection produces a self-limited disease, rarely leading to acute liver failure. The risk for symptomatic infection is related to the age of the patient. While children most commonly have either asymptomatic or unrecognized infection, more than 80% of adults exhibit symptoms of acute viral hepatitis, such as fatigue, malaise, nausea, vomiting, and anorexia.[8] Possible complications of hepatitis A include dehydration, electrolyte imbalance, bleeding, and rarely fulminant hepatitis. The prognosis depends on the age of the patient and the underlying liver condition. Approximately 10 to 15% of patients experience a relapse of symptoms during the 6 months following acute illness.
Diagnosis
History and Symptoms
Hepatitis A virus (HAV) infection can be either asymptomatic or symptomatic.[9] Symptoms of hepatitis A include fever, fatigue, loss of appetite, nausea, vomiting, abdominal pain, and jaundice.
Physical Examination
Hepatitis A virus infection is commonly associated with fever, jaundice, icteric sclera, abdominal tenderness, and hepatomegaly on physical examination. Other pertinent findings include rash, cervical lymphadenopathy, abdominal distension, ascites, and altered mental status.
Laboratory Findings
Hepatitis A cannot be differentiated from other types of viral hepatitis on the basis of clinical or epidemiologic features alone. Laboratory tests are required for its diagnosis. Serologic tests in hepatitis A virus (HAV) infection reveal elevated IgM anti-HAV in the acute phase (gold standard) in addition to an elevated IgG anti-HAV that remains elevated for the person's lifetime. Additional laboratory findings include the detection and sequencing of HAV RNA, an elevated direct bilirubin, and elevated liver enzymes. Liver biopsy has a minimal role in the diagnosis of HAV infection and it is only used when the diagnosis is unclear or when relapse is suspected.
CT
A CT scan is usually not indicated for the diagnosis of hepatitis A. However, it may be performed to exclude alternative diagnoses.
Ultrasound
An ultrasound of the abdomen may be performed in patients with hepatitis A to rule out other possible causes of hepatomegaly or chronic liver disease. Common ultrasound findings in acute hepatitis A include brightness of the portal vein walls and decreased echogenicity of the liver.
Treatment
Medical Therapy
There is no specific treatment for hepatitis A. Recovery from symptoms following infection may be slow and may take several weeks or months. Therapy is aimed at maintaining comfort and adequate nutritional balance, including replacement of fluids that are lost from vomiting and diarrhea.[10]
Surgery
Patients who develop fulminant hepatitis may require aggressive supportive therapy, and be transferred to a center capable of performing liver transplantation.
Primary Prevention
Two products are available for the prevention of HAV infection: hepatitis A vaccine and immune globulin (IG) for intramuscular (IM) administration. Administered IM in a 2-dose series at 0 and 6--12 months, these vaccines induce protective antibody levels in virtually all adults. By 1 month after the first dose, 94%--100% of adults have protective antibody levels; 100% of adults develop protective antibody after a second dose. IG is a sterile solution of concentrated immunoglobulins which, when administered IM before or within 2 weeks after exposure to HAV, is >85% effective in preventing HAV infections.[8] Vaccination is the most effective means of preventing HAV transmission among persons at risk for infection (e.g., MSM, illegal drug users, and persons with chronic liver disease). Because transmission of HAV during sexual activity probably results from fecal-oral contact, measures typically used to prevent the transmission of other sexually transmitted disease (e.g., use of condoms) do not prevent HAV transmission.[8]
Secondary Prevention
Until recently, an injection of immune globulin (IG) was the only recommended way to protect people after they have been exposed to hepatitis A virus. In June 2007, U.S. guidelines were revised to allow for hepatitis A vaccine to be used after exposure to prevent infection in healthy persons aged 1–40 years. Persons who recently have been exposed to hepatitis A virus (HAV) and who previously have not received hepatitis A vaccine should be administered a single dose of single-antigen vaccine or immunoglobulin (IG) (0.02 mL/kg) as soon as possible. Information about the relative efficacy of vaccine compared with immunoglobulin postexposure is limited, and no data are available for persons aged more than 40 years or those with underlying medical conditions. Therefore, decisions to use vaccine or immunoglobulin should take into account patient characteristics associated with more severe manifestations of hepatitis A, including older age and chronic liver disease.
References
- ↑ Ryan KJ, Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 541–4. ISBN 0838585299.
- ↑ Wasley A, Fiore A, Bell BP (2006). "Hepatitis A in the era of vaccination". Epidemiol Rev. 28: 101–11. doi:10.1093/epirev/mxj012. PMID 16775039.
- ↑ 3.0 3.1 Connor BA (2005). "Hepatitis A vaccine in the last-minute traveler". Am. J. Med. 118 (Suppl 10A): 58S–62S. doi:10.1016/j.amjmed.2005.07.018. PMID 16271543.
- ↑ Steffen R (2005). "Changing travel-related global epidemiology of hepatitis A". Am. J. Med. 118 (10): 46S–49S. doi:10.1016/j.amjmed.2005.07.016. PMID 16271541. Retrieved 2008-12-20. Unknown parameter
|month=ignored (help) - ↑ Jacobsen KH, Koopman JS (2005). "The effects of socioeconomic development on worldwide hepatitis A virus seroprevalence patterns". Int J Epidemiol. 34 (3): 600–9. doi:10.1093/ije/dyi062. PMID 15831565.
- ↑ Melnick JL (1995). "History and epidemiology of hepatitis A virus". J Infect Dis. 171 Suppl 1: S2–8. PMID [ 7876643 [ Check
|pmid=value (help). - ↑ "Hepatitis A Screening".
- ↑ 8.0 8.1 8.2 Sexually Transmitted Diseases Treatment Guidelines, 2010. Centers for Disease Control and Prevention. Recommendations and Reports December 17, 2010 / 59(RR12);1-110 [1]
- ↑ Krugman S, Giles JP (1970). "Viral hepatitis. New light on an old disease". JAMA : the Journal of the American Medical Association. 212 (6): 1019–29. PMID 4191502. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Hepatitis A. World Health Organization. Fact sheet N 328, updated June 2014. Accessed 07/28/2014.[2]
Pathophysiology
|
Hepatitis A |
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Hepatitis A On the Web |
|
American Roentgen Ray Society Images of Hepatitis A |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [7]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [8]
Overview
Hepatitis A is a liver disease caused by the hepatitis A virus (HAV). HAV has fecal-oral transmission and its infectivity peaks about 2-weeks before the onset of jaundice. Gross observation of the liver may show an enlarged and erythematous liver, while microscopically it may reveal lymphocyte infiltration and inflammation of the portal tracts.
Pathogenesis
- Hepatitis A is a liver disease caused by the HAV that can affect anyone.
- HAV is acquired by mouth (through fecal-oral transmission) and replicates in the liver. After 10-12 days, the virus is present in blood and is excreted via the biliary system into the feces.
- Peak titers occur during the 2 weeks before onset of illness. Although virus is present in serum, its concentration is several orders of magnitude less than in feces. Virus excretion begins to decline at the onset of clinical illness, and has decreased significantly by 7–10 days after onset of symptoms.
- Peak infectivity occurs during the 2-week period before onset of jaundice, or elevation of liver enzymes, when the concentration of virus in stool is highest. [1][2]
- Children may excrete virus longer than adults.
- Chronic shedding of HAV in feces does not occur; however, shedding can occur in persons who have relapsing illness.[4]
- The virus is resistant to detergent, acid, solvents, drying, and temperatures up to 60ºC. It can survive for months in salt water. Common-source outbreaks, such as water or restaurants are typical.
- HAV can be inactivated by chlorine treatment, formalin, peracetic acid, beta-propiolactone, and UV radiation.
Transmission
The virus spreads by the fecal-oral route and infections often occur in conditions of poor sanitation. Hepatitis A can be transmitted by the parenteral route but very rarely by blood, and blood products. Today transmission of the virus through blood is rare, however, some risk groups such as IV drug users and their care takers are still infected by this route.[5]
Food-borne outbreaks are not uncommon,[6] and ingestion of shellfish cultivated in polluted water is associated with a high risk of infection.[7]
Direct Transmission
- Eating food prepared by someone who has HAV and who did not properly wash their hands after using the bathroom
- Having anal or oral sex with someone who has HAV
- Not washing hands after changing a diaper (young children often are asymptomatic)
Indirect Transmission
- Eating uncooked food that is HAV-contaminated
- Eating cooked food that is not heated to 185°F (85°C) for 1 minute after being contaminated with HAV
- Drinking contaminated water: This is a common route of infection in underdeveloped countries. Chlorinated water, such as tap water in developed countries, kills HAV
Gross Pathology
Gross observation of the liver with acute hepatitis A may include:
- Enlarged liver
- Erythematous surface
Microscopic Pathology
Virus-induced cytopathology may not be responsible for the pathologic changes seen in HAV infection, as liver disease may result primarily from immune mechanisms. Antigen-specific T-lymphocytes are responsible for the destruction of infected hepatocytes.[8]
Common histologic findings include:
- Inflammatory infiltrates (lymphocytes)
- Necrosis and apoptosis of hepatocytes
- The hepatocyte inflammation may have the following patterns:
- Multifocal
- Panacinar
- Inflammation of portal tracts
Rarely, patients with acute viral hepatitis A develop features of cholestasis.[8]
Confluent hepatic necrosis may lead to fulminant hepatitis and death in 30-60% of cases. Death appears to be inevitable when necrosis involves more than 65-80% of the total hepatocyte fraction. In patients who survive an episode of acute fulminant hepatic failure, neither functional nor pathologic sequelae are common, despite the widespread necrosis.[8]
During the recovery stage, cell regeneration is prominent. The damaged hepatic tissue is usually restored within 8 to 12 weeks.[8]
Shown below is a video depicting the pathological findings in viral hepatitis. Click on the arrow to view the video. {{#ev:youtube|_hXvbpSxFZw}}
References
- ↑ Skinh j P, Mathiesen LR, Kryger P, M ller AM. Faecal excretion of hepatitis A virus in patients with symptomatic hepatitis A infection. Scand J Gastroenterol 1981;16:1057-9.
- ↑ 2.0 2.1 Tassopoulos NC, Papaevangelou GJ, Ticehurst JR, Purcell RH (1986). "Fecal excretion of Greek strains of hepatitis A virus in patients with hepatitis A and in experimentally infected chimpanzees". The Journal of Infectious Diseases. 154 (2): 231–7. PMID 3014009. Retrieved 2012-02-28. Unknown parameter
|month=ignored (help) - ↑ Skinh j P, Mathiesen LR, Kryger P, M ller AM. Faecal excretion of hepatitis A virus in patients with symptomatic hepatitis A infection. Scand J Gastroenterol 1981;16:1057-9.
- ↑ Sjogren MH, Tanno H, Fay O, Sileoni S, Cohen BD, Burke DS, Feighny RJ (1987). "Hepatitis A virus in stool during clinical relapse". Annals of Internal Medicine. 106 (2): 221–6. PMID 3026213. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Melnick JL (1995). "History and epidemiology of hepatitis A virus". J Infect Dis. 171 Suppl 1: S2–8. PMID [ 7876643 [ Check
|pmid=value (help). - ↑ Brundage SC, Fitzpatrick AN (2006). "Hepatitis A". Am Fam Physician. 73 (12): 2162–8. PMID 16848078.
- ↑ Lees D (2000). "Viruses and bivalve shellfish". Int. J. Food Microbiol. 59 (1–2): 81–116. doi:10.1016/S0168-1605(00)00248-8. PMID 10946842.
- ↑ 8.0 8.1 8.2 8.3 "Hepatitis A" (PDF).
Epidemiology and Demographics
|
Hepatitis A |
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Hepatitis A On the Web |
|
American Roentgen Ray Society Images of Hepatitis A |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [9]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [10]
Overview
The incidence of hepatitis A varies among eras, countries and even cities within the same country. In recent years it has been noted a shift in prevalence, what was once a disease more prevalent in children, is today predominant in adults. In the United States, the incidence of hepatitis A in 2011 was 0.4 cases per 100,000 population. In recent years, the rates of hepatitis A have been similar among all age groups. After the introduction of the HAV vaccine, historic differences in rates of hepatitis A among racial/ethnic populations have also narrowed. In developed countries, elimination of historic geographic differences in incidence rates has also occurred. In developing countries with very poor sanitary conditions and hygienic practices, most children (90%) are infected with the hepatitis A virus before the age of 10.
Incidence
Prevaccine Era
- Before vaccine licensure during 1995-1996, hepatitis A incidence was primarily cyclic, with peaks occurring every 10-15 years.
- In the United States, during 1980-1995, approximately 22,000-36,000 hepatitis A cases were reported annually to CDC (rate: 9.0-14.5 cases per 100,000 population), but incidence models indicate that the number of infections was substantially higher.[1][2]
Postvaccine Era
- There were 1,398 reported cases of acute HAV in 2011, representing an estimated 2,700 (1,650- 4,370) actual acute cases.[3]
- The number of acute hepatitis A cases reported in the United States declined by approximately 53%, from 2,979 in 2007 to 1,398 in 2011.[3]
- Of the 50 states that reported hepatitis A cases in 2011, 24 states had rates below the national rate.[3]
- The rate of acute hepatitis A in the United States declined from 1.0 case per 100,000 population in 2007 to 0.4 cases per 100,000 population in 2011.[3]
- In 2011, the case rate ranged from no cases in New Hampshire and North Dakota to 1.2 cases per 100,000 population in Arizona.[3]
Improvement in Sanitation
- The resistance of the virus allows it to survive in urban sewage. Accordingly, outbreaks of the disease occur in overcrowded areas where there is poor sanitation. Improvements made throughout the years have decreased the incidence of the infection in new infants, which has led to an increasing number of adults with hepatitis A.
Age
Prevaccine Era
- The reported incidence of hepatitis A was highest among children aged 5-14 years, with approximately one third of reported cases involving children aged <15 years.[4]
- Because young children frequently have unrecognized or asymptomatic infection, a relatively smaller proportion of infections among children than adults are detected by routine disease surveillance. Incidence models indicate that during 1980-1999, the majority of HAV infections occurred among children aged <10 years, and the highest incidence was among those aged 0-4 years.[1]
Postvaccine Era
- In recent years, rates of hepatitis A have been similar among all age groups.[5]
Race
Prevaccine Era
- Hepatitis A rates among American Indians and Alaska Natives were more than five times higher than rates in other racial/ethnic populations, and rates among Hispanics were approximately three times higher than rates among non-Hispanics.[6][7][8][9]
Postvaccine Era
- The historic differences in the rates of hepatitis A among racial/ethnic populations have narrowed in the vaccine era.
- Recent rates among American Indians and Alaska Natives represent a 99% decline compared with the prevaccine era and are now approximately the same or lower than those of other racial/ethnic populations.[9]
- Rates among Hispanics also declined 87% during this period, from 20.6 cases per 100,000 population during 1990-1997 to 2.7 per 100,000 in 2004, but remain higher than those for non-Hispanics.[5][10]
Developed Countries
Prevaccine Era
- Since the 1960s, the highest hepatitis A rates and the majority of cases occurred in a limited number of states and counties concentrated in the western and southwestern United States.[5]
- Despite year-to-year fluctuations, rates in these areas consistently remained above the national average. In 11 states (Alaska, Arizona, California, Idaho, Nevada, New Mexico, Oklahoma, Oregon, South Dakota, Utah, and Washington) with consistently elevated rates, representing 22% of the U.S. population, average annual hepatitis A incidence was >20 cases per 100,000 during 1987-1997 (twice the national average of approximately 10 cases per 100,000 population); cases among residents of these states accounted for an average of 50% of reported cases.[11]
- An additional 18% of cases occurred among residents of six states (Arkansas, Colorado, Missouri, Montana, Texas, and Wyoming) with average annual rates above (but less than twice) the national average during this time.
- The majority of U.S. cases of hepatitis A resulted from person-to-person transmission of HAV during communitywide outbreaks.[12][13]
- The most frequently reported source of infection (in 12%-26% of cases) was household or sexual contact with a person with hepatitis A.[14]
- For approximately 50% of persons with hepatitis A, no source was identified for their infection.
Postvaccine Era
- There has been an elimination of the historic geographic differences in the incidence rates of hepatitis A in the United States. Since 2001, rates of hepatitis A in states where vaccination was recommended, have been approximately equal to the rest of the United States.[15] In recent years, counties with higher rates have varied from year to year and have been distributed throughout the country.[5]
- In developed countries with good sanitary and hygienic conditions, infection rates are low. Disease may occur among adolescents and adults in high-risk groups, such as injecting-drug users, men who have sex with men, people traveling to areas of high endemicity, and in isolated populations such as closed religious communities.[16]
Developing Countries
Areas with High Levels of Infection
- In developing countries with very poor sanitary conditions and hygienic practices, most children (90%) have been infected with the hepatitis A virus before the age of 10. Those infected in childhood do not experience any noticeable symptoms.
- Epidemics are uncommon because older children and adults are generally immune. Symptomatic disease rates in these areas are low and outbreaks are rare.[16]
Areas with Intermediate Levels of Infection
- In developing countries, countries with transitional economies and regions where sanitary conditions are variable, children often escape infection in early childhood. Ironically, these improved economic and sanitary conditions may lead to a higher susceptibility in older age groups and higher disease rates, as infections occur in adolescents and adults, and large outbreaks can occur.[16]
References
- ↑ 1.0 1.1 Armstrong GL, Bell BP (2002). "Hepatitis A virus infections in the United States: model-based estimates and implications for childhood immunization". Pediatrics. 109 (5): 839–45. PMID 11986444. Retrieved 2012-02-28. Unknown parameter
|month=ignored (help) - ↑ CDC. Hepatitis surveillance. Report no. 61. Atlanta, GA: US Department of Health and Human Services, CDC. 2006
- ↑ 3.0 3.1 3.2 3.3 3.4 "Hepatitis A Epidemics".
- ↑ CDC. Hepatitis surveillance. Report no. 61. Atlanta, GA: US Department of Health and Human Services, CDC. 2006
- ↑ 5.0 5.1 5.2 5.3 Wasley A, Samandari T, Bell BP (2005). "Incidence of hepatitis A in the United States in the era of vaccination". JAMA : the Journal of the American Medical Association. 294 (2): 194–201. doi:10.1001/jama.294.2.194. PMID 16014593. Retrieved 2012-02-28. Unknown parameter
|month=ignored (help) - ↑ CDC. Hepatitis surveillance. Report no. 61. Atlanta, GA: US Department of Health and Human Services, CDC. 2006
- ↑ Shaw FE, Shapiro CN, Welty TK, Dill W, Reddington J, Hadler SC (1990). "Hepatitis transmission among the Sioux Indians of South Dakota". American Journal of Public Health. 80 (9): 1091–4. PMC 1404852. PMID 2166446. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Bulkow LR, Wainwright RB, McMahon BJ, Middaugh JP, Jenkerson SA, Margolis HS (1993). "Secular trends in hepatitis A virus infection among Alaska Natives". The Journal of Infectious Diseases. 168 (4): 1017–20. PMID 8376812. Retrieved 2012-02-28. Unknown parameter
|month=ignored (help) - ↑ 9.0 9.1 Bialek SR, Thoroughman DA, Hu D, Simard EP, Chattin J, Cheek J, Bell BP (2004). "Hepatitis A incidence and hepatitis a vaccination among American Indians and Alaska Natives, 1990-2001". American Journal of Public Health. 94 (6): 996–1001. PMC 1448379. PMID 15249305. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ CDC. Hepatitis surveillance. Report no. 61. Atlanta, GA: US Department of Health and Human Services, CDC. 2006
- ↑ CDC. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1999;48(No. RR-12):1-37
- ↑ Bell BP, Shapiro CN, Alter MJ, Moyer LA, Judson FN, Mottram K, Fleenor M, Ryder PL, Margolis HS (1998). "The diverse patterns of hepatitis A epidemiology in the United States-implications for vaccination strategies". The Journal of Infectious Diseases. 178 (6): 1579–84. PMID 9815207. Retrieved 2012-02-28. Unknown parameter
|month=ignored (help) - ↑ CDC. Communitywide outbreaks of hepatitis A. Hepatitis surveillance. Report no. 51. Atlanta, GA: US Department of Health and Human Services, CDC; 1987:6-8.
- ↑ Shapiro CN, Coleman PJ, McQuillan GM, Alter MJ, Margolis HS (1992). "Epidemiology of hepatitis A: seroepidemiology and risk groups in the USA". Vaccine. 10 Suppl 1: S59–62. PMID 1476001.
|access-date=requires|url=(help) - ↑ CDC. Hepatitis surveillance. Report no. 61. Atlanta, GA: US Department of Health and Human Services, CDC. 2006
- ↑ 16.0 16.1 16.2 Hepatitis A. World Health Organization. Fact sheet N 328, updated June 2014. Accessed 07/28/2014.[3]
Risk Factors
|
Hepatitis A |
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Hepatitis A On the Web |
|
American Roentgen Ray Society Images of Hepatitis A |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [11]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [12]
Overview
Common risk factors in the development of hepatitis A include travel to endemic areas, poor sanitation, and intravenous drug users.
Risk Factors
Travelers
- Persons from developed countries who travel to developing countries are at substantial risk for acquiring hepatitis A.[1] Such persons include tourists, immigrants, and their children returning to their country of origin to visit friends or relatives, military personnel, missionaries, and others who work or study abroad in countries that have high or intermediate endemicity of hepatitis A.
- Hepatitis A remains one of the most common vaccine-preventable diseases acquired during travel.
- The risk might be higher among travelers staying in areas with poor hygienic conditions, varies according to the region and the length of stay, and appears to be increased even among travelers who reported observing protective measures and staying in urban areas or luxury hotels.
- In the United States, children account for approximately 50% of reported travel-related cases.[2]
- Travelers who acquire hepatitis A during their trips also might transmit to others on their return.
MSM
- Hepatitis A outbreaks among MSM (men who have sex with men) have been reported frequently.
- Cyclic outbreaks have occurred in urban areas in the United States, Canada, Europe, and Australia and can occur in the context of an outbreak in the larger community.[3][4][5][6][7]
- Seroprevalence surveys have not consistently demonstrated an elevated prevalence of anti-HAV compared with a similarly aged general population.[8][9]
- Since 1996, ACIP has recommended hepatitis A vaccination of MSM.[10] Although precise data are lacking, vaccine coverage appears to be low. [5]
Users of Injection and Noninjection Drugs
- During the last 2 decades, outbreaks have been reported with increasing frequency among users of injection and noninjection drugs in Australia, Europe, and North America.[4][11][12][13][14]
- In the United States, outbreaks have frequently involved users of injected and non-injected methamphetamine, who have accounted for up to 48% of reported cases during outbreaks.[13][15] Cross-sectional serologic surveys have demonstrated that injection-drug users have a higher prevalence of anti-HAV than the general U.S. population.[8][16]
- Transmission among injection-drug users probably occurs through both percutaneous and fecal-oral routes.[15]
- Since 1996, ACIP has recommended hepatitis A vaccination of users of illicit drugs, but vaccine coverage data are not available.[17]
Persons with Clotting-Factor Disorders
- During 1992-1993, outbreaks of hepatitis A were reported in Europe among persons with clotting-factor disorders who had been administered solvent-detergent-treated, "high-purity" factor VIII concentrates that presumably had been contaminated from plasma donors incubating hepatitis A.[18]
- In the United States, data from one serologic study suggested that persons with hemophilia might be at increased risk for HAV infection.[19]
- HAV is resistant to solvent-detergent treatment.[20]
Persons Working with Nonhuman Primates
- Outbreaks of hepatitis A have been reported among persons working with nonhuman primates that are susceptible to HAV infection, including Old and New World species.[21][22] Primates that were infected were those that had been born in the wild, not those born and raised in captivity.
Risk for Severe Adverse Consequences of Hepatitis A Among Persons with Chronic Liver Disease
- Although not at increased risk for HAV infection, persons with chronic liver disease are at increased risk for fulminant hepatitis A.[23][24][25] Death certificate data indicate a higher prevalence of chronic liver disease among persons who died of fulminant hepatitis A compared with persons who died of other causes.[26]
Risk for Hepatitis A in Other Groups and Settings
Food-Service Establishments and Food Handlers
- Foodborne hepatitis A outbreaks are recognized relatively infrequently in the United States. Outbreaks typically are associated with contamination of food during preparation by an HAV-infected food handler; a single infected food handler can transmit HAV to dozens or even hundreds of persons.[27][28][29][30][31] However, the majority of food handlers with hepatitis A do not transmit HAV. Food handlers are not at increased risk for hepatitis A because of their occupation. However, among the approximately 40,000 adults with hepatitis A reported during 1992--2000 for whom an occupation was known, 8% were identified as food handlers, reflecting the large number of persons employed in the food service industry.[27]
- Evaluating HAV-infected food handlers is a common and labor-intensive task for public health departments. In a 1992 common-source outbreak involving 43 persons, the estimated total medical and disease control cost was approximately $800,000.[32]
- Outbreaks associated with food, especially green onions and other raw produce, that has been contaminated before reaching a food-service establishment have been recognized increasingly in recent years. Low attack rates are common, and outbreaks often have been recognized in association with a single restaurant in which no infected food handler was identified on subsequent investigation.[33][34][35]
Child Care Centers
- Outbreaks among children attending child care centers and persons employed at these centers have been recognized since the 1970s, but their frequency has decreased as overall hepatitis A incidence among children has declined in recent years.[36][37][38]
- Because infection among children is typically mild or asymptomatic, outbreaks often are identified only when adult contacts (typically parents) become ill.[37][39]
- Poor hygiene among children who wear diapers and the handling and changing of diapers by staff contribute to the spread of HAV infection; outbreaks rarely occur in child care centers in which care is provided only to children who are toilet trained.
- Although child care centers might have been the source of outbreaks of hepatitis A in certain communities, disease in child care centers more commonly reflects extended transmission from the community. Despite the occurrence of outbreaks when HAV is introduced into child care centers, results of serologic surveys do not indicate a substantially increased prevalence of HAV infection among staff at child care centers compared with prevalence among control populations.[40]
Health-Care Institutions
- Nosocomial HAV transmission is rare.
- Outbreaks have occasionally been observed in neonatal intensive-care units because of infants acquiring infection from transfused blood and subsequently transmitting hepatitis A to other infants and staff.[41][42][43]
- Outbreaks of hepatitis A caused by transmission from adult patients to health-care workers are typically associated with fecal incontinence, although the majority of hospitalized patients who have hepatitis A are admitted after onset of jaundice, when they are beyond the point of peak infectivity.[44][45]
- Data from serologic surveys of health-care workers have not indicated an increased prevalence of HAV infection in these groups compared with that in control populations.[46]
Institutions for Persons with Developmental Disabilities
- Historically, HAV infection was highly endemic in institutions for persons with developmental disabilities.[47] As fewer children have been institutionalized and as conditions in institutions have improved, the incidence and prevalence of HAV infection have decreased, although outbreaks can occur in these settings.
Schools
- In the United States, the occurrence of cases of hepatitis A in elementary or secondary schools typically reflects disease acquisition in the community.
- Child-to-child disease transmission in the school setting is uncommon; if multiple cases occur among children at a school, the possibility of a common source of infection should be investigated.[48][49]
Workers Exposed to Sewage
- Data from serologic studies conducted outside the United States indicate that workers who had been exposed to sewage had a possible elevated risk for HAV infection; however, these analyses did not control for other risk factors (e.g., socioeconomic status).[50][51][52]
- In published reports of three serologic surveys conducted among U.S. wastewater workers and appropriate comparison populations, no substantial or consistent increase in the prevalence of anti-HAV was identified among wastewater workers.[53][54][55] No work-related instances of HAV transmission have been reported among wastewater workers in the United States.
References
- ↑ Steffen R, Kane MA, Shapiro CN, Billo N, Schoellhorn KJ, van Damme P (1994). "Epidemiology and prevention of hepatitis A in travelers". JAMA : the Journal of the American Medical Association. 272 (11): 885–9. PMID 8078167. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ CDC. Hepatitis surveillance. Report no. 61. Atlanta, GA: US Department of Health and Human Services, CDC. 2006.
- ↑ Nainan OV, Armstrong GL, Han XH, Williams I, Bell BP, Margolis HS (2005). "Hepatitis a molecular epidemiology in the United States, 1996-1997: sources of infection and implications of vaccination policy". The Journal of Infectious Diseases. 191 (6): 957–63. doi:10.1086/427992. PMID 15717272. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ 4.0 4.1 Bell BP, Shapiro CN, Alter MJ, Moyer LA, Judson FN, Mottram K, Fleenor M, Ryder PL, Margolis HS (1998). "The diverse patterns of hepatitis A epidemiology in the United States-implications for vaccination strategies". The Journal of Infectious Diseases. 178 (6): 1579–84. PMID 9815207. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ 5.0 5.1 Cotter SM, Sansom S, Long T, Koch E, Kellerman S, Smith F, Averhoff F, Bell BP (2003). "Outbreak of hepatitis A among men who have sex with men: implications for hepatitis A vaccination strategies". The Journal of Infectious Diseases. 187 (8): 1235–40. doi:10.1086/374057. PMID 12696002. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ CDC. Hepatitis A among homosexual men-United States, Canada, and Australia.MMWR 1992;41:155, 161-4.
- ↑ Friedman MS, Blake PA, Koehler JE, Hutwagner LC, Toomey KE (2000). "Factors influencing a communitywide campaign to administer hepatitis A vaccine to men who have sex with men". American Journal of Public Health. 90 (12): 1942–6. PMC 1446451. PMID 11111274. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ 8.0 8.1 Villano SA, Nelson KE, Vlahov D, Purcell RH, Saah AJ, Thomas DL (1997). "Hepatitis A among homosexual men and injection drug users: more evidence for vaccination". Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America. 25 (3): 726–8. PMID 9314468. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ Katz MH, Hsu L, Wong E, Liska S, Anderson L, Janssen RS (1997). "Seroprevalence of and risk factors for hepatitis A infection among young homosexual and bisexual men". The Journal of Infectious Diseases. 175 (5): 1225–9. PMID 9129091. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ CDC. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1996;45(No. RR-15):1-30.
- ↑ Harkess J, Gildon B, Istre GR (1989). "Outbreaks of hepatitis A among illicit drug users, Oklahoma, 1984-87". American Journal of Public Health. 79 (4): 463–6. PMC 1349976. PMID 2929804. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Hutin YJ, Bell BP, Marshall KL, Schaben CP, Dart M, Quinlisk MP, Shapiro CN (1999). "Identifying target groups for a potential vaccination program during a hepatitis A communitywide outbreak". American Journal of Public Health. 89 (6): 918–21. PMC 1508638. PMID 10358687. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ 13.0 13.1 Vong S, Fiore AE, Haight DO, Li J, Borgsmiller N, Kuhnert W, Pinero F, Boaz K, Badsgard T, Mancini C, Nainan OV, Wiersma S, Bell BP (2005). "Vaccination in the county jail as a strategy to reach high risk adults during a community-based hepatitis A outbreak among methamphetamine drug users". Vaccine. 23 (8): 1021–8. doi:10.1016/j.vaccine.2004.07.038. PMID 15620475. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ CDC. Hepatitis A among drug abusers. MMWR 1988;37:297-300, 305
- ↑ 15.0 15.1 Hutin YJ, Sabin KM, Hutwagner LC, Schaben L, Shipp GM, Lord DM, Conner JS, Quinlisk MP, Shapiro CN, Bell BP (2000). "Multiple modes of hepatitis A virus transmission among methamphetamine users". American Journal of Epidemiology. 152 (2): 186–92. PMID 10909956. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ Ivie K, Spruill C, Bell B. Prevalence of hepatitis A virus infection among illicit drug users, 1993--1994 [Abstract no. A010]. Antiviral Therapy 2000;5(Suppl 1):A.7.
- ↑ CDC. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1996;45(No. RR-15):1-30.
- ↑ Mannucci PM, Gdovin S, Gringeri A, Colombo M, Mele A, Schinaia N, Ciavarella N, Emerson SU, Purcell RH (1994). "Transmission of hepatitis A to patients with hemophilia by factor VIII concentrates treated with organic solvent and detergent to inactivate viruses. The Italian Collaborative Group". Annals of Internal Medicine. 120 (1): 1–7. PMID 7504424. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Mah MW, Royce RA, Rathouz PJ, Wang JG, White GC, Janco RL, Hanna WT, Davis PC, Johnson CA, Poon MC (1994). "Prevalence of hepatitis A antibodies in hemophiliacs: preliminary results from the Southeastern Delta Hepatitis Study". Vox Sanguinis. 67 Suppl 1: 21–2, discussion 23. PMID 8091730.
|access-date=requires|url=(help) - ↑ Soucie JM, Robertson BH, Bell BP, McCaustland KA, Evatt BL (1998). "Hepatitis A virus infections associated with clotting factor concentrate in the United States". Transfusion. 38 (6): 573–9. PMID 9661691. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Hinthorn DR, Foster MT, Bruce HL, Aach RD (1974). "An outbreak of chimpanzee associated hepatitis". Journal of Occupational Medicine. : Official Publication of the Industrial Medical Association. 16 (6): 388–91. PMID 4836166. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Dienstag JL, Davenport FM, McCollum RW, Hennessy AV, Klatskin G, Purcell RH (1976). "Nonhuman primate-associated viral hepatitis type A. Serologic evidence of hepatitis A virus infection". JAMA : the Journal of the American Medical Association. 236 (5): 462–4. PMID 180303. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Akriviadis EA, Redeker AG (1989). "Fulminant hepatitis A in intravenous drug users with chronic liver disease". Annals of Internal Medicine. 110 (10): 838–9. PMID 2712463. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G, Ferraro T, Concia E (1998). "Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C". The New England Journal of Medicine. 338 (5): 286–90. doi:10.1056/NEJM199801293380503. PMID 9445408. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ Keeffe EB (1995). "Is hepatitis A more severe in patients with chronic hepatitis B and other chronic liver diseases?". The American Journal of Gastroenterology. 90 (2): 201–5. PMID 7847285. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Williams I, Bell B, Kaluba J, Shapiro C. Association between chronic liver disease and death from hepatitis A, United States, 1989--92 [Abstract no. A39]. IX Triennial International Symposium on Viral Hepatitis and Liver Disease. Rome, Italy, April 21--25, 1996
- ↑ 27.0 27.1 Fiore AE (2004). "Hepatitis A transmitted by food". Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America. 38 (5): 705–15. doi:10.1086/381671. PMID 14986256. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ Weltman AC, Bennett NM, Ackman DA, Misage JH, Campana JJ, Fine LS, Doniger AS, Balzano GJ, Birkhead GS (1996). "An outbreak of hepatitis A associated with a bakery, New York, 1994: the 1968 "West Branch, Michigan' outbreak repeated". Epidemiology and Infection. 117 (2): 333–41. PMC 2271694. PMID 8870631. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Lowry PW, Levine R, Stroup DF, Gunn RA, Wilder MH, Konigsberg C (1989). "Hepatitis A outbreak on a floating restaurant in Florida, 1986". American Journal of Epidemiology. 129 (1): 155–64. PMID 2910057. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Massoudi MS, Bell BP, Paredes V, Insko J, Evans K, Shapiro CN (1999). "An outbreak of hepatitis A associated with an infected foodhandler". Public Health Reports (Washington, D.C. : 1974). 114 (2): 157–64. PMC 1308455. PMID 10199718.
|access-date=requires|url=(help) - ↑ Latham RH, Schable CA (1982). "Foodborne hepatitis A at a family reunion use of IgM-specific hepatitis a serologic testing". American Journal of Epidemiology. 115 (5): 640–5. PMID 6282115. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Dalton CB, Haddix A, Hoffman RE, Mast EE (1996). "The cost of a food-borne outbreak of hepatitis A in Denver, Colo". Archives of Internal Medicine. 156 (9): 1013–6. PMID 8624166. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ Amon JJ, Devasia R, Xia G, Nainan OV, Hall S, Lawson B, Wolthuis JS, Macdonald PD, Shepard CW, Williams IT, Armstrong GL, Gabel JA, Erwin P, Sheeler L, Kuhnert W, Patel P, Vaughan G, Weltman A, Craig AS, Bell BP, Fiore A (2005). "Molecular epidemiology of foodborne hepatitis a outbreaks in the United States, 2003". The Journal of Infectious Diseases. 192 (8): 1323–30. doi:10.1086/462425. PMID 16170748. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ Dentinger CM, Bower WA, Nainan OV, Cotter SM, Myers G, Dubusky LM, Fowler S, Salehi ED, Bell BP (2001). "An outbreak of hepatitis A associated with green onions". The Journal of Infectious Diseases. 183 (8): 1273–6. doi:10.1086/319688. PMID 11262211. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ Wheeler C, Vogt TM, Armstrong GL, Vaughan G, Weltman A, Nainan OV, Dato V, Xia G, Waller K, Amon J, Lee TM, Highbaugh-Battle A, Hembree C, Evenson S, Ruta MA, Williams IT, Fiore AE, Bell BP (2005). "An outbreak of hepatitis A associated with green onions". The New England Journal of Medicine. 353 (9): 890–7. doi:10.1056/NEJMoa050855. PMID 16135833. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ CDC. Hepatitis surveillance. Report no. 61. Atlanta, GA: US Department of Health and Human Services, CDC. 2006.
- ↑ 37.0 37.1 Hadler SC, Webster HM, Erben JJ, Swanson JE, Maynard JE (1980). "Hepatitis A in day-care centers. A community-wide assessment". The New England Journal of Medicine. 302 (22): 1222–7. doi:10.1056/NEJM198005293022203. PMID 6245363. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ Venczel LV, Desai MM, Vertz PD, England B, Hutin YJ, Shapiro CN, Bell BP (2001). "The role of child care in a community-wide outbreak of hepatitis A". Pediatrics. 108 (5): E78. PMID 11694662. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ Shapiro CN, Hadler SC (1991). "Hepatitis A and hepatitis B virus infections in day-care settings". Pediatric Annals. 20 (8): 435–41. PMID 1945541. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Jackson LA, Stewart LK, Solomon SL, et al. Risk of infection with hepatitis A, B or C, cytomegalovirus, varicella or measles among child care providers. Pediatr Infect Dis J 1996;15:584-9.
- ↑ Rosenblum LS, Villarino ME, Nainan OV, Melish ME, Hadler SC, Pinsky PP, Jarvis WR, Ott CE, Margolis HS (1991). "Hepatitis A outbreak in a neonatal intensive care unit: risk factors for transmission and evidence of prolonged viral excretion among preterm infants". The Journal of Infectious Diseases. 164 (3): 476–82. PMID 1651359. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ Klein BS, Michaels JA, Rytel MW, Berg KG, Davis JP (1984). "Nosocomial hepatitis A. A multinursery outbreak in Wisconsin". JAMA : the Journal of the American Medical Association. 252 (19): 2716–21. PMID 6492350. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Noble RC, Kane MA, Reeves SA, Roeckel I (1984). "Posttransfusion hepatitis A in a neonatal intensive care unit". JAMA : the Journal of the American Medical Association. 252 (19): 2711–5. PMID 6492349. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Goodman RA (1985). "Nosocomial hepatitis A". Annals of Internal Medicine. 103 (3): 452–4. PMID 4026088. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Papaevangelou GJ, Roumeliotou-Karayannis AJ, Contoyannis PC (1981). "The risk of nosocomial hepatitis A and B virus infections from patients under care without isolation precaution". Journal of Medical Virology. 7 (2): 143–8. PMID 6267188.
|access-date=requires|url=(help) - ↑ Gibas A, Blewett DR, Schoenfeld DA, Dienstag JL (1992). "Prevalence and incidence of viral hepatitis in health workers in the prehepatitis B vaccination era". American Journal of Epidemiology. 136 (5): 603–10. PMID 1442723. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Szmuness W, Purcell RH, Dienstag JL, Stevens CE (1977). "Antibody to hepatitis A antigen in institutionalized mentally retarded patients". JAMA : the Journal of the American Medical Association. 237 (16): 1702–5. PMID 139479. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Hutin YJ, Pool V, Cramer EH, Nainan OV, Weth J, Williams IT, Goldstein ST, Gensheimer KF, Bell BP, Shapiro CN, Alter MJ, Margolis HS (1999). "A multistate, foodborne outbreak of hepatitis A. National Hepatitis A Investigation Team". The New England Journal of Medicine. 340 (8): 595–602. doi:10.1056/NEJM199902253400802. PMID 10029643. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ Niu MT, Polish LB, Robertson BH, Khanna BK, Woodruff BA, Shapiro CN, Miller MA, Smith JD, Gedrose JK, Alter MJ (1992). "Multistate outbreak of hepatitis A associated with frozen strawberries". The Journal of Infectious Diseases. 166 (3): 518–24. PMID 1323618. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ Lerman Y, Chodik G, Aloni H, Ribak J, Ashkenazi S (1999). "Occupations at increased risk of hepatitis A: a 2-year nationwide historical prospective study". American Journal of Epidemiology. 150 (3): 312–20. PMID 10430237. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ Glas C, Hotz P, Steffen R (2001). "Hepatitis A in workers exposed to sewage: a systematic review". Occupational and Environmental Medicine. 58 (12): 762–8. PMC 1740082. PMID 11706141. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ Poole CJ, Shakespeare AT (1993). "Should sewage workers and carers for people with learning disabilities be vaccinated for hepatitis A?". BMJ (Clinical Research Ed.). 306 (6885): 1102. PMC 1677505. PMID 8388287. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Trout D, Mueller C, Venczel L, Krake A (2000). "Evaluation of occupational transmission of hepatitis A virus among wastewater workers". Journal of Occupational and Environmental Medicine / American College of Occupational and Environmental Medicine. 42 (1): 83–7. PMID 10652693. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ Weldon M, VanEgdom MJ, Hendricks KA, Regner G, Bell BP, Sehulster LM (2000). "Prevalence of antibody to hepatitis A virus in drinking water workers and wastewater workers in Texas from 1996 to 1997". Journal of Occupational and Environmental Medicine / American College of Occupational and Environmental Medicine. 42 (8): 821–6. PMID 10953820. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help) - ↑ Venczel L, Brown S, Frumkin H, Simmonds-Diaz J, Deitchman S, Bell BP (2003). "Prevalence of hepatitis A virus infection among sewage workers in Georgia". American Journal of Industrial Medicine. 43 (2): 172–8. doi:10.1002/ajim.10174. PMID 12541272. Retrieved 2012-02-29. Unknown parameter
|month=ignored (help)
Screening
|
Hepatitis A |
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Hepatitis A On the Web |
|
American Roentgen Ray Society Images of Hepatitis A |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [13]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [14]
Overview
There is no recommended screening guideline for Hepatitis A infection in the general population. However, screening is often performed in young adult migrants, refugees, and adopted children. The detection of hepatitis A virus (HAV) antibodies in the blood is used to screen for hepatitis A. Anti-HAV IgG remains elevated after acute disease.[1][2]
Screening
A positive anti-HAV IgG result demonstrates that the person is immune to hepatitis A due to: [1]
- Previous HAV infection, or
- Hepatitis A vaccination
A negative test demonstrates that the person:[1]
- Has never been infected with HAV
- Has never been vaccinated against HAV
- Is vulnerable to the HAV infection
References
- ↑ 1.0 1.1 1.2 "Hepatitis A Screening".
- ↑ Fishbain JT, Eckart RE, Harner KC, Hospenthal DR (2002). "Empiric immunization versus serologic screening: developing a cost-effective strategy for the use of hepatitis A immunization in travelers". J Travel Med. 9 (2): 71–5. PMID 12044273.
Causes
|
Hepatitis A |
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Hepatitis A On the Web |
|
American Roentgen Ray Society Images of Hepatitis A |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [15]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [16]
Overview
Hepatitis A infection is caused by the hepatitis A virus.
Taxonomy
Viruses; ssRNA viruses; ssRNA virus; positive-strand viruses; Picornavirales; Picornaviridae; Hepatovirus
Biology
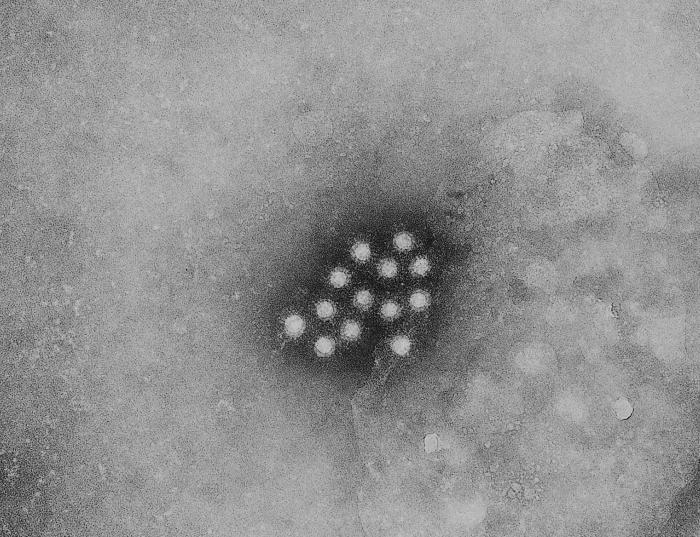 |
Hepatitis A virus (HAV) is a 27 nm, non-enveloped, icosahedral, ssRNA virus with a single serovar. So far, 4 different genotypes have been identified in hepatitis A viruses, all belonging to the same serotype.[2]
HAV has no lipid envelope and is stable when excreted from the infected liver to the bile to enter the gastrointestinal tract. It has been found to survive in experimentally contaminated fresh water, seawater, wastewater, soils, marine sediment, live oysters, and creme-filled cookies.[3]
HAV is extremely resistant to degradation by environmental conditions, a property that allows its maintenance and spread within populations.[3]
Genome
The HAV genome is encoded in 7474 nucleotides, which are divided into three regions:
- 5' untranslated region, with 742 nucleotides
- Single reading frame encoding a 2227 amino acid polypeptide, with 6681 nucleotides
- 3' noncoding region, with 63 nucleotides
The reading frame encodes a polypeptide that is processed by a viral protease. This process takes place cotranslationally, and leads to the formation of:
Tropism
Similarly to other hepatitis viruses, the hepatitis A virus demonstrates tropism for the liver cells. The hepatocytes represent the predominant site for viral replication.[4]
Natural Reservoir
- Humans are the only natural reservoir of the virus.[5]
- There are no insect or animal vectors.[5]
- A chronic HAV carrier state has not been reported.[5]
References
- ↑ "http://phil.cdc.gov/phil/details.asp". External link in
|title=(help) - ↑ Lemon SM, Jansen RW, Brown EA (1992). "Genetic, antigenic and biological differences between strains of hepatitis A virus". Vaccine. 10 Suppl 1: S40–4. PMID 1335657.
- ↑ 3.0 3.1 "Hepatitis A" (PDF).
- ↑ Lemon SM (1997). "Type A viral hepatitis: epidemiology, diagnosis, and prevention". Clin Chem. 43 (8 Pt 2): 1494–9. PMID 9265900.
- ↑ 5.0 5.1 5.2 "Hepatitis A".
Differential Diagnosis

Editor-In-Chief: C. Michael Gibson, M.S., M.D. [17]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [18]
Overview
Hepatitis A must be differentiated from other diseases that cause fever, nausea, vomiting, jaundice, hepatomegaly, icteric sclera, elevated ALT, AST, and PCR such as other viral hepatitis, alcoholic hepatitis, and autoimmune hepatitis.
Differentiating Hepatitis A from other Diseases
Shown below is a table that summarizes the findings that differentiate hepatitis A from other conditions that cause fever, nausea, vomiting, jaundice, hepatomegaly, and icteric sclera.[1]
| Disease | Findings |
|---|---|
| Viral Hepatitis B | Symptoms are similar to the ones of hepatitis A; however, hepatitis B is a life-threatening condition caused by the hepatitis B virus, that may lead to the development of cirrhosis and liver cancer. Serologic testing will help differentiate these two conditions. |
| Viral Hepatitis C | Symptoms are similar to the ones of hepatitis A; however, hepatitis C, caused by the hepatitis C virus, can cause acute and/or chronic hepatitis. Serologic testing will help differentiate these two conditions. |
| Viral Hepatitis D | Symptoms are similar to the ones of hepatitis A; however, hepatitis D is a serious liver disease caused by infection with the hepatitis D virus. Hepatitis D only occurs among people who are infected with the Hepatitis B virus (HBV). It may complicate into cirrhosis and hepatocellular carcinoma. Serologic testing will help differentiate these two conditions. |
| Viral Hepatitis E | Symptoms are similar to the ones of hepatitis A; however, hepatitis E, caused by the hepatitis E virus, may complicate, in rare cases, into chronic hepatitis and liver failure. Serologic testing will help differentiate these two conditions. |
| Alcoholic Hepatitis | Symptoms are similar to the ones of hepatitis A; however, alcoholic hepatitis is related to the excessive use of alcohol. Alcoholic hepatitis presents more often with ascites. Alcoholic hepatitis often leads to cirrhosis and liver failure, if alcohol use is not decreased. Serologic testing will help differentiate these two conditions. Also laboratory results show ALT<AST in alcoholic hepatitis (inverse from hepatitis A, in which ALT>AST). |
| Autoimmune Hepatitis | Autoimmune hepatitis occurs when the body's immune system attacks the hepatocytes. It often affects young females and may present with signs of acute hepatitis or chronic liver disease. Serologic testing will help differentiate these two conditions. |
Differential diagnosis of jaundice as one of symptoms of hepatitis A are: [2][3][4][5][6]
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
- ↑ "Hepatitis A".
- ↑ Fargo MV, Grogan SP, Saguil A (2017). "Evaluation of Jaundice in Adults". Am Fam Physician. 95 (3): 164–168. PMID 28145671.
- ↑ Leevy CB, Koneru B, Klein KM (1997). "Recurrent familial prolonged intrahepatic cholestasis of pregnancy associated with chronic liver disease". Gastroenterology. 113 (3): 966–72. PMID 9287990.
- ↑ Hov JR, Boberg KM, Karlsen TH (2008). "Autoantibodies in primary sclerosing cholangitis". World J. Gastroenterol. 14 (24): 3781–91. PMC 2721433. PMID 18609700.
- ↑ Bond LR, Hatty SR, Horn ME, Dick M, Meire HB, Bellingham AJ (1987). "Gall stones in sickle cell disease in the United Kingdom". Br Med J (Clin Res Ed). 295 (6592): 234–6. PMC 1247079. PMID 3115390.
- ↑ Malakouti M, Kataria A, Ali SK, Schenker S (2017). "Elevated Liver Enzymes in Asymptomatic Patients - What Should I Do?". J Clin Transl Hepatol. 5 (4): 394–403. doi:10.14218/JCTH.2017.00027. PMC 5719197. PMID 29226106.
Natural History, Complications & Prognosis
Natural History
|
Hepatitis A |
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Hepatitis A On the Web |
|
American Roentgen Ray Society Images of Hepatitis A |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [19]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [20]
Overview
Hepatitis A is caused by infection with hepatitis A virus (HAV) which has an incubation period of approximately 28 days. HAV infection produces a self-limited disease, rarely leading to acute liver failure. The risk for symptomatic infection is related to the age of the patient. While children most commonly have either asymptomatic or unrecognized infection, more than 80% of adults exhibit symptoms of acute viral hepatitis, such as fatigue, malaise, nausea, vomiting, and anorexia.[1] Possible complications of hepatitis A include dehydration, electrolyte imbalance, bleeding, and rarely fulminant hepatitis. The prognosis depends on the age of the patient and the underlying liver condition. Approximately 10 to 15% of patients experience a relapse of symptoms during the 6 months following acute illness.
Natural History
Hepatitis A is caused by the hepatitis A virus (HAV) infection. Unlike other types of hepatitis, HAV infection is always acute. The virus replicates in the liver and is shed in high concentrations in feces from 2 weeks before to 1 week after the onset of clinical illness. It is primarily spread through fecal-oral transmission, commonly after ingesting food or water that is contaminated with the virus. The infected patients have a peak infectivity during the 2 week period before onset of jaundice or elevation of liver enzymes.[2]
The likelihood of developing symptoms with HAV infection increases with age. Fewer than 10% of infections among children aged 0-4 years result in jaundice; this percentage increases to 30%-40% among children aged 5-9 years, 60%-80% among youths aged 10-17 years, and 80%-90% among adults aged ≥18 years.[3]
When signs and symptoms occur, they typically last less than 2 months, although 10-15% of symptomatic persons have prolonged or relapsing disease lasting up to 6 months.[4] HAV infection is usually acute and self-limited. The rare cases of fulminant hepatitis are more common among patients with previous liver disease, such as chronic hepatitis C.[5]
The clinical manifestations commonly start after a 30 day incubation period. The disease manifests abruptly, with the following symptoms:[6]
One week after symptom onset, patients experience:
- Jaundice
- Pruritus
- Dark urine
- Acholic stool
The initial symptoms commonly diminish after the onset of jaundice, which is usually more intense on its second week.
Complications
Possible complications of hepatitis A include:
- Severe dehydration
- Electrolyte imbalance
- Bleeding
- After the first month of the disease, there may be low immunity, with a consequent increased risk for opportunistic infections.
- Fulminant hepatitis
- Death (particularly in elderly and adults with chronic liver disease, such as hepatitis C)
Prognosis
- Adults are often confined to bed and minimal activity for about 4 weeks and have to stop their work for one to three months or longer.
- Many adults take up to 36 months and occasionally longer to recover entirely.
- It is common for recovering patients to experience occasional "off" days, during which they need to rest more.
- Approximately 15% of people diagnosed with hepatitis A may experience one or more symptomatic relapse(s) for up to 24 months after contracting this disease.
- The United States Centers for Disease Control and Prevention (CDC) reported that the mortality rate of hepatitis A in 2010 was 0.03 deaths per 100,000 population.[7]
- The case-fatality rate for HAV infection increases with age: 1.8% for adults older than 50 years of age, compared with 0.6% for persons below 50 years. The case-fatality rate is also increased among persons with chronic liver disease, who are at increased risk for acute liver failure.[8]
References
- ↑ Sexually Transmitted Diseases Treatment Guidelines, 2010. Centers for Disease Control and Prevention. Recommendations and Reports December 17, 2010 / 59(RR12);1-110 [4]
- ↑ Tassopoulos NC, Papaevangelou GJ, Ticehurst JR, Purcell RH (1986). "Fecal excretion of Greek strains of hepatitis A virus in patients with hepatitis A and in experimentally infected chimpanzees". The Journal of Infectious Diseases. 154 (2): 231–7. PMID 3014009. Retrieved 2012-02-28. Unknown parameter
|month=ignored (help) - ↑ Armstrong GL, Bell BP (2002). "Hepatitis A virus infections in the United States: model-based estimates and implications for childhood immunization". Pediatrics. 109 (5): 839–45. PMID 11986444. Retrieved 2012-02-28. Unknown parameter
|month=ignored (help) - ↑ Glikson M, Galun E, Oren R, Tur-Kaspa R, Shouval D (1992). "Relapsing hepatitis A. Review of 14 cases and literature survey". Medicine. 71 (1): 14–23. PMID 1312659. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G; et al. (1998). "Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C." N Engl J Med. 338 (5): 286–90. doi:10.1056/NEJM199801293380503. PMID 9445408.
- ↑ Lednar WM, Lemon SM, Kirkpatrick JW, Redfield RR, Fields ML, Kelley PW (1985). "Frequency of illness associated with epidemic hepatitis A virus infections in adults". Am J Epidemiol. 122 (2): 226–33. PMID 3860002.
- ↑ "Hepatitis A".
- ↑ Williams I, Bell B, Kaluba J, Shapiro C. Association between chronic liver disease and death from hepatitis A, United States, 1989--92 [abstract no. A39]. IX Triennial International Symposium on Viral Hepatitis and Liver Disease. Rome, Italy, April 21--25, 1996.
Diagnosis
{{#ask:Used To Diagnose::Hepatitis A |?Sort Order |format=list |headers=hide |link=none |sep= | |template=MedicalTestQuery |sort=Sort Order }}
Treatment
{{#ask:Used To Treat::Hepatitis A |?Sort Order |format=list |headers=hide |link=none |sep= | |template=MedicalTreatmentQuery |sort=Sort Order }} {{#ask:Prevents::Hepatitis A |?Sort Order |intro= | |format=list |headers=hide |link=none |sep= | |template=MedicalTreatmentQuery2 |sort=Sort Order }}
{{#set:On The Web = Most recent articles }}
{{#set:On The Web = Most cited articles }}
{{#set:On The Web = Review articles }}
{{#set:On The Web = CME Programs }}
{{#set:On The Web = Powerpoint slides }}
{{#set:On The Web = Images }}
{{#set:On The Web = American Roentgen Ray Images }}
{{#set:On The Web = Ongoing Trials at Clinical Trials.gov }}
{{#set:On The Web = US National Guidelines Clearinghouse }}
{{#set:On The Web = NICE Guidance }}
{{#set:On The Web = FDA on Hepatitis A }}
{{#set:On The Web = CDC on Hepatitis A }}
{{#set:On The Web = Hepatitis A in the news }}
{{#set:On The Web = Blogs on Hepatitis A }}
{{#set:On The Web = Directions to Hospitals treating Hepatitis A }}
{{#set:On The Web = Risk calculators and risk factors for Hepatitis A }}
- Pages using duplicate arguments in template calls
- CS1 maint: Extra text: authors list
- CS1 maint: Multiple names: authors list
- Pages with citations using unsupported parameters
- CS1 errors: PMID
- Pages using citations with accessdate and no URL
- Pages with reference errors
- CS1 errors: external links
- CS1 maint: Explicit use of et al.
- Foodborne illnesses
- Hepatitis
- Picornaviruses
- Viral diseases
- Mature chapter
- Disease
- Emergency mdicine
- Up-To-Date
- Infectious disease
- Hepatology
- Gastroenterology
- Needs overview