Elotuzumab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Martin Nino [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Elotuzumab is a SLAMF7-directed immunostimulatory antibody that is FDA approved for the treatment of patients with multiple myeloma (in combination with lenalidomide and dexamethasone) who have received one to three prior therapies.. Common adverse reactions include fatigue, diarrhea, pyrexia, constipation, cough, peripheral neuropathy, nasopharyngitis, upper respiratory tract infection, decreased appetite, pneumonia (20%).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Elotuzumab is indicated in combination with lenalidomide and dexamethasone for the treatment of patients with multiple myeloma who have received one to three prior therapies.
Dosage
- Recommended Dosing
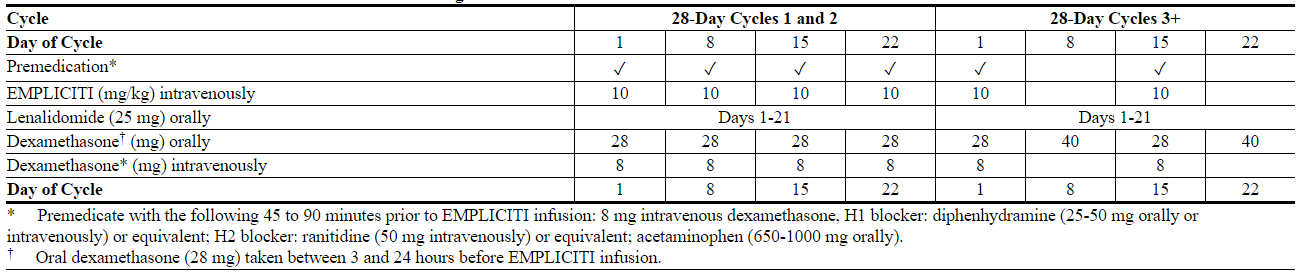
- The recommended dosage of Elotuzumab is 10 mg/kg administered intravenously every week for the first two cycles and every 2 weeks thereafter in conjunction with the recommended dosing of lenalidomide and low-dose dexamethasone as described below. Continue treatment until disease progression or unacceptable toxicity.
- Refer to the dexamethasone and lenalidomide prescribing information for additional information.
- Patients must be premedicated before each dose of Elotuzumab.
- Administer dexamethasone as follows:
- On days that Elotuzumab is administered, give dexamethasone 28 mg orally between 3 and 24 hours before Elotuzumab plus 8 mg intravenously between 45 and 90 minutes before Elotuzumab.
- On days that Elotuzumab is not administered but a dose of dexamethasone is scheduled (Days 8 and 22 of cycle 3 and all subsequent cycles), give 40 mg orally.
- The recommended dosing is presented in Table 1.
- Table 1: Recommended Dosing Schedule of Elotuzumab in Combination with Lenalidomide and Dexamethasone
EMPLICITI: Elotuzumab's Brand name
- Premedication
- When Elotuzumab is used in combination with lenalidomide, divide dexamethasone into an oral and intravenous dose and administer as shown in Table 1.
- Other Medications
- In addition to dexamethasone, complete administration of the following medications 45 to 90 minutes prior to Elotuzumab infusion:
- - H1 blocker: diphenhydramine (25-50 mg orally or intravenously) or equivalent H1 blocker.
- - H2 blocker: ranitidine (50 mg intravenously or 150 mg orally) or equivalent H2 blocker.
- - Acetaminophen (650-1000 mg orally).
- Dose Modifications
- If the dose of one drug in the regimen is delayed, interrupted, or discontinued, the treatment with the other drugs may continue as scheduled. However, if dexamethasone is delayed or discontinued, base the decision whether to administer Elotuzumab on clinical judgment (i.e., risk of hypersensitivity).
- If a Grade 2 or higher infusion reaction occurs during Elotuzumab administration, interrupt the infusion and institute appropriate medical and supportive measures. Upon resolution to Grade 1 or lower, restart Elotuzumab at 0.5 mL per minute and gradually increase at a rate of 0.5 mL per minute every 30 minutes as tolerated to the rate at which the infusion reaction occurred. Resume the escalation regimen if there is no recurrence of the infusion reaction (see Table 2).
- In patients who experience an infusion reaction, monitor vital signs every 30 minutes for 2 hours after the end of the Elotuzumab infusion. If the infusion reaction recurs, stop the Elotuzumab infusion and do not restart on that day. Severe infusion reactions may require permanent discontinuation of Elotuzumab therapy and emergency treatment.
- Dose delays and modifications for dexamethasone and lenalidomide should be performed as recommended in their Prescribing Information.
- Table 2: Infusion Rate for Elotuzumab

Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Elotuzumab in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Elotuzumab in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness have not been established in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Elotuzumab in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Elotuzumab in pediatric patients.
Contraindications
There are no contraindications to Elotuzumab. Because Elotuzumab is indicated for use in combination with lenalidomide and dexamethasone, healthcare providers should consult the prescribing information of these products for a complete description of contraindications before starting therapy.
Warnings
Infusion Reactions
Elotuzumab can cause infusion reactions. Infusion reactions were reported in approximately 10% of patients treated with Elotuzumab with lenalidomide and dexamethasone in the randomized trial in multiple myeloma. All reports of infusion reaction were Grade 3 or lower. Grade 3 infusion reactions occurred in 1% of patients. The most common symptoms of an infusion reaction included fever, chills, and hypertension. Bradycardia and hypotension also developed during infusions.
In the trial, 5% of patients required interruption of the administration of Elotuzumab for a median of 25 minutes due to infusion reactions, and 1% of patients discontinued due to infusion reactions. Of the patients who experienced an infusion reaction, 70% (23/33) had them during the first dose.
Administer premedication consisting of dexamethasone, antihistamines (H1 and H2 blockers) and acetaminophen prior to Elotuzumab infusion.
Interrupt Elotuzumab infusion for Grade 2 or higher infusion reactions and institute appropriate medical management.
Infections
In a clinical trial of patients with multiple myeloma (N=635), infections were reported in 81.4% of patients in the Elotuzumab combined with lenalidomide and dexamethasone (E-Ld) arm and 74.4% in lenalidomide and dexamethasone (Ld). Grade 3 to 4 infections were noted in 28% and 24.3% of E-Ld- and Ld-treated patients, respectively. Discontinuations due to infections occurred in 3.5% of E-Ld-treated and 4.1% of Ld-treated patients. Fatal infections were reported in 2.5% and 2.2% of E-Ld- and Ld-treated patients.
Opportunistic infections were reported in 22% of patients in the E-Ld arm and 12.9% of patients in the Ld arm. Fungal infections occurred in 9.7% of patients in the E-Ld arm and 5.4% of patients in the Ld arm. Herpes zoster was reported in 13.5% of patients treated with E-Ld and 6.9% of patients treated with Ld. Monitor patients for development of infections and treat promptly.
Second Primary Malignancies
In a clinical trial of patients with multiple myeloma (N=635), invasive second primary malignancies (SPM) have been observed in 9.1% of patients treated with E-Ld and 5.7% of patients treated with Ld. The rate of hematologic malignancies were the same between E-Ld and Ld treatment arms (1.6%). Solid tumors were reported in 3.5% and 2.2% of E-Ld- and Ld-treated patients, respectively. Skin cancer was reported in 4.4% and 2.8% of patients treated with E-Ld and Ld, respectively. Monitor patients for the development of second primary malignancies.
Hepatotoxicity
Elevations in liver enzymes (aspartate transaminase/alanine transaminase [AST/ALT] greater than 3 times the upper limit, total bilirubin greater than 2 times the upper limit, and alkaline phosphatase less than 2 times the upper limit) consistent with hepatotoxicity were reported in 2.5% and 0.6% of E-Ld- and Ld-treated patients in a clinical trial of patients with multiple myeloma (N=635). Two patients experiencing hepatotoxicity were not able to continue treatment; however, 6 out of 8 patients had resolution and were able to continue treatment. Monitor liver enzymes periodically. Stop Elotuzumab upon Grade 3 or higher elevation of liver enzymes. After return to baseline values, continuation of treatment may be considered.
Interference with Determination of Complete Response
Elotuzumab is a humanized IgG kappa monoclonal antibody that can be detected on both the serum protein electrophoresis (SPEP) and immunofixation (IFE) assays used for the clinical monitoring of endogenous M-protein. This interference can impact the determination of complete response and possibly relapse from complete response in patients with IgG kappa myeloma protein.
Adverse Reactions
Clinical Trials Experience
The following adverse reactions are described in detail in other sections of the label:
- Infusion reaction
- Infections
- Second Primary Malignancies
- Hepatotoxicity
- Interference with determination of complete response
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described in this section are based on a randomized, open-label clinical trial in patients with previously treated multiple myeloma. In this study, Elotuzumab 10 mg/kg was administered with lenalidomide and dexamethasone. For adverse reaction evaluation, Elotuzumab combined with lenalidomide and dexamethasone was compared with lenalidomide and dexamethasone alone.
The mean age of the population was 66 years and 57% of patients were 65 years of age or older. Sixty percent (60%) of the population were male, 84% were white, 10% were Asian, and 4% were black. The Eastern Cooperative Oncology Group (ECOG) performance status was 0 in 47%, 1 in 44%, and 2 in 9% of patients.
These data reflect exposure of 318 patients to Elotuzumab and 317 to control with a median number of cycles of 19 for Elotuzumab and 14 for control.
Serious adverse reactions were reported in 65.4% of patients treated on the Elotuzumab arm and 56.5% for patients treated on the control arm. The most frequent serious adverse reactions in the Elotuzumab arm compared to the control arm were: pneumonia (15.4% vs. 11%), pyrexia (6.9% vs. 4.7%), respiratory tract infection (3.1% vs. 1.3%), anemia (2.8% vs. 1.9%), pulmonary embolism (3.1% vs. 2.5%), and acute renal failure (2.5% vs. 1.9%).
The proportion of patients who discontinued any component of the treatment regimen due to adverse reactions as listed below was similar for both treatment arms; 6.0% for patients treated on the Elotuzumab arm and 6.3% for patients treated on the control.
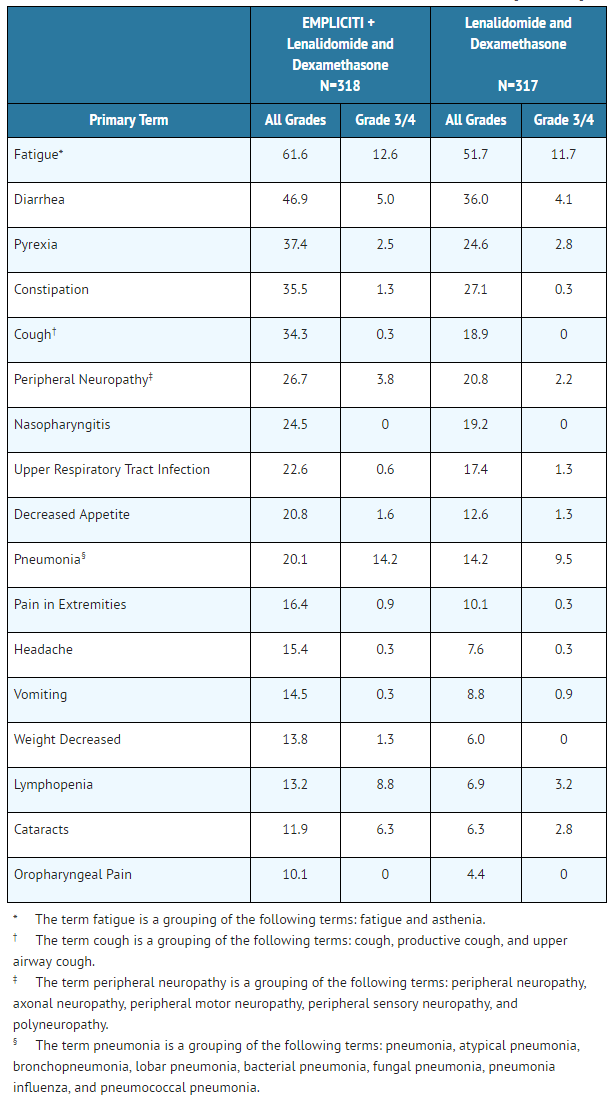
Adverse reactions occurring at a frequency of 10% or higher in the Elotuzumab arm and 5% or higher than the lenalidomide and dexamethasone arm for the randomized trial in multiple myeloma are presented in Table 4.
- Table 4: Adverse Reactions with a 10% or Higher Incidence for Elotuzumab-Treated Patients and a 5% or Higher Incidence than Lenalidomide and Dexamethasone-Treated Patients [All Grades]
EMPLICITI: Elotuzumab's Brand name
Other clinically important adverse reactions reported in patients treated with Elotuzumab that did not meet the criteria for inclusion in Table 4 but occurred at a frequency of 5% or greater in the Elotuzumab group and at a frequency at least twice the control rate for the randomized trial in multiple myeloma are listed below:
- General disorders and administration site conditions: chest pain
- Immune system disorders: hypersensitivity
- Nervous system disorders: hypoesthesia
- Psychiatric disorders: mood altered
- Skin and subcutaneous tissue disorders: night sweats
Laboratory abnormalities worsening from baseline and occurring at a frequency of 10% or higher in the Elotuzumab group and 5% or higher than the lenalidomide and dexamethasone group (criteria met for all Grades or Grade 3/4) for the randomized trial in multiple myeloma are presented in Table 5.
- Table 5:Laboratory Abnormalities Worsening from Baseline and with a 10% or Higher Incidence for Elotuzumab-Treated Patients and a 5% Higher Incidence than Lenalidomide and Dexamethasone-Treated Patients [Criteria met for All Grades or Grade 3/4]

EMPLICITI: Elotuzumab's Brand name
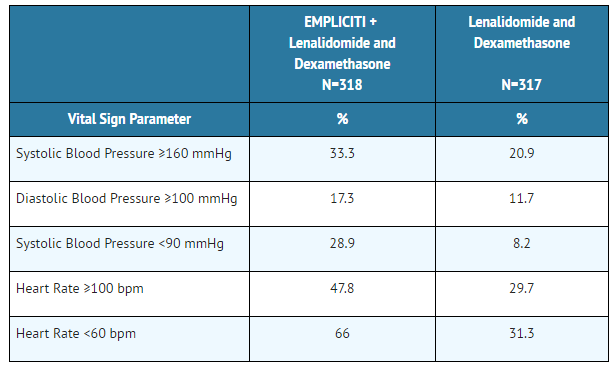
Vital sign abnormalities were assessed by treatment arm for the randomized trial in multiple myeloma and are presented in Table 6. Percentages are based on patients who had at least one on-treatment vital sign abnormality any time during the course of therapy.
- Table 6: Vital Sign Abnormalities
EMPLICITI: Elotuzumab's Brand name
Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity to Elotuzumab.
Of 390 patients across four clinical studies who were treated with Elotuzumab and evaluable for the presence of anti-product antibodies, 72 patients (18.5%) tested positive for treatment-emergent anti-product antibodies by an electrochemiluminescent (ECL) assay. In 63 (88%) of these 72 patients, anti-product antibodies occurred within the first 2 months of the initiation of Elotuzumab treatment. Anti-product antibodies resolved by 2 to 4 months in 49 (78%) of these 63 patients. Neutralizing antibodies were detected in 19 of 299 patients in the randomized trial in multiple myeloma. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of incidence of antibodies to Elotuzumab with the incidences of antibodies to other products may be misleading.
Postmarketing Experience
There is limited information regarding Elotuzumab Postmarketing Experience in the drug label.
Drug Interactions
No formal drug-drug interaction studies have been conducted with Elotuzumab. However, Elotuzumab is used in combination with lenalidomide and dexamethasone. Refer to the prescribing information for those products for important drug-drug interactions.
- Laboratory Test Interference
- Elotuzumab may be detected in the SPEP and serum immunofixation assays of myeloma patients and could interfere with correct response classification. A small peak in the early gamma region on SPEP that is IgGƙ on serum immunofixation may potentially be attributed to Elotuzumab, particularly in patients whose endogenous myeloma protein is IgA, IgM, IgD, or lambda light chain restricted. This interference can impact the determination of complete response and possibly relapse from complete response in patients with IgG kappa myeloma protein.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): N There are no studies with Elotuzumab with pregnant women to inform any drug associated risks. Animal reproduction studies have not been conducted with elotuzumab.
Elotuzumab is administered in combination with lenalidomide and dexamethasone. Lenalidomide can cause embryo-fetal harm and is contraindicated for use in pregnancy. Refer to the lenalidomide and dexamethasone prescribing information for additional information. Lenalidomide is only available through a REMS program.
The background risk in the U.S. general population of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Elotuzumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Elotuzumab during labor and delivery.
Nursing Mothers
There is no information on the presence of Elotuzumab in human milk, the effect on the breast-fed infant, or the effect on milk production. Because of the potential for serious adverse reactions in breast-fed infants from elotuzumab administered with lenalidomide/dexamethasone, breastfeeding is not recommended. Refer to the lenalidomide and dexamethasone prescribing information for additional information.
Pediatric Use
Safety and effectiveness have not been established in pediatric patients.
Geriatic Use
Of the 646 patients across treatment groups in the randomized trial in multiple myeloma, 57% were 65 years of age or older; the number of patients 65 years or older was similar between treatment groups. No overall differences in efficacy or safety were observed between patients 65 years or older and younger patients (less than 65 years of age).
Gender
There is no FDA guidance on the use of Elotuzumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Elotuzumab with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Elotuzumab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Elotuzumab in patients with hepatic impairment.
Females of Reproductive Potential and Males
- Pregnancy Testing
- Refer to the lenalidomide labeling for pregnancy testing requirements prior to initiating treatment in females of reproductive potential.
- When Elotuzumab is used with lenalidomide, there is a risk of fetal harm, including severe life-threatening human birth defects associated with lenalidomide, and the need to follow requirements regarding pregnancy avoidance, including testing.
- Contraception
- Refer to the lenalidomide labeling for contraception requirements prior to initiating treatment in females of reproductive potential and males.
- Lenalidomide is present in the blood and semen of patients receiving the drug. Refer to the lenalidomide full prescribing information for requirements regarding contraception and the prohibitions against blood and/or sperm donation due to presence and transmission in blood and/or semen and for additional information.
Immunocompromised Patients
There is no FDA guidance one the use of Elotuzumab in patients who are immunocompromised.
Administration and Monitoring
Administration
Administer the entire Elotuzumab infusion with an infusion set and a sterile, nonpyrogenic, low-protein-binding filter (with a pore size of 0.2-1.2 micrometer) using an automated infusion pump. Initiate Elotuzumab infusion at a rate of 0.5 mL per minute. The infusion rate may be increased in a stepwise fashion as described in Table 2 if no infusion reactions develop. The maximum infusion rate should not exceed 2 mL per minute.
Adjust the infusion rate following a Grade 2 or higher infusion reaction.
In patients who have received 4 cycles of Elotuzumab treatment, the infusion rate may be increased to a maximum of 5 mL/min.
Do not mix Elotuzumab with, or administer as an infusion with, other medicinal products. No physical or biochemical compatibility studies have been conducted to evaluate the coadministration of Elotuzumab with other agents.
- Reconstitution and Preparation
- Calculation of Dose
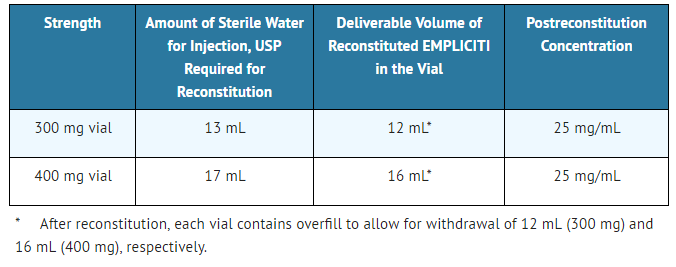
- - Calculate the dose (mg) and determine the number of vials needed for the 10 mg/kg dosage based on patient weight.
- - Determine the volume of sterile water for injection (SWFI) needed for reconstitution as shown in Table 3.
- Table 3: Reconstitution Instructions for Elotuzumab
EMPLICITI: Elotuzumab's Brand name
- Reconstitution
- - Aseptically reconstitute each Elotuzumab vial with a syringe of adequate size and an 18-gauge or smaller needle (e.g., 17, 16, 15). A slight back pressure may be experienced during administration of the Sterile Water for Injection, USP, which is considered normal.
- - Hold the vial upright and swirl the solution by rotating the vial to dissolve the lyophilized cake. Invert the vial a few times in order to dissolve any powder that may be present on top of the vial or the stopper. Avoid vigorous agitation. DO NOT SHAKE. The lyophilized powder should dissolve in less than 10 minutes.
- - After the remaining solids are completely dissolved, allow the reconstituted solution to stand for 5 to 10 minutes. The reconstituted preparation results in a colorless to slightly yellow, clear to slightly opalescent solution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard the solution if any particulate matter or discoloration is observed.
- Dilution
- - Once the reconstitution is completed, withdraw the necessary volume for the calculated dose from each vial, up to a maximum of 16 mL from 400 mg vial and 12 mL from 300 mg vial.
- - Further dilute with 230 mL of either 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP, into an infusion bag made of polyvinyl chloride or polyolefin.
- - The volume of 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP can be adjusted so as not to exceed 5 mL/kg of patient weight at any given dose of Elotuzumab.
Complete the Elotuzumab infusion within 24 hours of reconstitution of the Elotuzumab lyophilized powder. If not used immediately, the infusion solution may be stored under refrigeration conditions: 2ºC to 8ºC (36ºF-46ºF) and protected from light for up to 24 hours (a maximum of 8 hours of the total 24 hours can be at room temperature, 20°C to 25°C [68°F-77°F], and room light).
Monitoring
There is limited information regarding Elotuzumab Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Elotuzumab and IV administrations.
Overdosage
The dose of Elotuzumab at which severe toxicity occurs is not known. Elotuzumab does not appear to be removed by dialysis as determined in a study of patients with renal impairment.
In case of overdosage, monitor patients closely for signs or symptoms of adverse reactions and institute appropriate symptomatic treatment.
Pharmacology
Elotuzumab?
| |
| Therapeutic monoclonal antibody | |
| Source | zu |
| Target | SLAMF7 (CD319) |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 145.5 kg/mol |
| Pharmacokinetic data | |
| Bioavailability | 100% (IV) |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
X(US) X (used with lenalidomide) |
| Legal status |
?(US) |
| Routes | IV |
Mechanism of Action
Elotuzumab is a humanized IgG1 monoclonal antibody that specifically targets the SLAMF7 (Signaling Lymphocytic Activation Molecule Family member 7) protein. SLAMF7 is expressed on myeloma cells independent of cytogenetic abnormalities. SLAMF7 is also expressed on Natural Killer cells, plasma cells, and at lower levels on specific immune cell subsets of differentiated cells within the hematopoietic lineage.
Elotuzumab directly activates Natural Killer cells through both the SLAMF7 pathway and Fc receptors. Elotuzumab also targets SLAMF7 on myeloma cells and facilitates the interaction with Natural Killer cells to mediate the killing of myeloma cells through antibody-dependent cellular cytotoxicity (ADCC). In preclinical models, the combination of elotuzumab and lenalidomide resulted in enhanced activation of Natural Killer cells that was greater than the effects of either agent alone and increased anti-tumor activity in vitro and in vivo.
Structure
Elotuzumab is a humanized recombinant monoclonal antibody directed to SLAMF7, a cell surface glycoprotein. Elotuzumab consists of the complementary determining regions (CDR) of the mouse antibody, MuLuc63, grafted onto human IgG1 heavy and kappa light chain frameworks. Elotuzumab is produced in NS0 cells by recombinant DNA technology. Elotuzumab has a theoretical mass of 148.1 kDa for the intact antibody.
Elotuzumab is a sterile, nonpyrogenic, preservative-free lyophilized powder that is white to off-white, whole or fragmented cake in single-dose vials. Elotuzumab for Injection is supplied as 300 mg per vial and 400 mg per vial and requires reconstitution with Sterile Water for Injection, USP (13 mL and 17 mL, respectively) to obtain a solution with a concentration of 25 mg/mL. After reconstitution, each vial contains overfill to allow for withdrawal of 12 mL (300 mg) and 16 mL (400 mg). The reconstituted solution is colorless to slightly yellow, clear to slightly opalescent. Prior to intravenous infusion, the reconstituted solution is diluted with 230 mL of either 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP.
Each 300 mg single-dose vial of Elotuzumab also contains the following inactive ingredients: citric acid monohydrate (2.44 mg), polysorbate 80 (3.4 mg), sodium citrate (16.6 mg), and sucrose (510 mg).
Each 400 mg single-dose vial of Elotuzumab also contains the following inactive ingredients: citric acid monohydrate (3.17 mg), polysorbate 80 (4.4 mg), sodium citrate (21.5 mg), and sucrose (660 mg).
Pharmacodynamics
- Elotuzumab does not prolong the QT interval to any clinically relevant extent in combination with lenalidomide and dexamethasone at the recommended dose or as monotherapy (at a dose 2 times the recommended dose).
Pharmacokinetics
Elotuzumab exhibits nonlinear pharmacokinetics (PK) resulting in greater than proportional increases in area under the concentration-time curve (AUC) indicative of target-mediated clearance. The administration of the recommended 10 mg/kg Elotuzumab regimen in combination with lenalidomide/dexamethasone is predicted to result in geometric mean (CV%) steady-state trough concentrations of 194 μg/mL (52%).
- Elimination: The clearance of elotuzumab decreased from a geometric mean (CV%) of 17.5 (21.2%) to 5.8 (31%) mL/day/kg with an increase in dose from 0.5 (i.e., 0.05 times the recommended dosage) to 20 mg/kg (i.e., 2 times the recommended dosage). Based on a population PK model, when elotuzumab is given in combination with lenalidomide and dexamethasone, approximately 97% of the maximum steady-state concentration is predicted to be eliminated with a geometric mean (CV%) of 82.4 (48%) days.
- Specific Populations
- Clinically significant differences were not observed in the pharmacokinetics of elotuzumab based on age (37-88 years), gender, race, baseline LDH, albumin, renal impairment ranging from mild to severe (creatinine clearance (CLcr) 15 to 89 mL/min) renal impairment, end-stage renal disease (CLcr less than 15 mL/min) with or without hemodialysis, and mild (NCI-CTEP) hepatic impairment. The pharmacokinetics of elotuzumab in patients with moderate to severe hepatic impairment is unknown.
- Body weight: The clearance of elotuzumab increased with increasing body weight supporting a weight-based dose.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or mutagenicity data are available for elotuzumab in animals or humans. Fertility studies have not been performed for elotuzumab.
Clinical Studies
The efficacy and safety of Elotuzumab in combination with lenalidomide and dexamethasone were evaluated in a randomized, open-label trial in patients with multiple myeloma who had received one to three prior therapies and had documented progression following their most recent therapy.
Eligible patients were randomized in a 1:1 ratio to receive either Elotuzumab in combination with lenalidomide and low-dose dexamethasone or lenalidomide and low-dose dexamethasone. Treatment was administered in 4-week cycles until disease progression or unacceptable toxicity. Elotuzumab 10 mg/kg was administered intravenously each week for the first 2 cycles and every 2 weeks thereafter. Prior to Elotuzumab infusion, dexamethasone was administered as a divided dose: an oral dose of 28 mg and an intravenous dose of 8 mg. In the control group and on weeks without Elotuzumab, dexamethasone 40 mg was administered as a single oral dose weekly. Lenalidomide 25 mg was taken orally once daily for the first 3 weeks of each cycle. Assessment of tumor response was conducted every 4 weeks.
A total of 646 patients were randomized to receive treatment: 321 to Elotuzumab in combination with lenalidomide and low-dose dexamethasone and 325 to lenalidomide and low-dose dexamethasone.
Demographics and baseline disease characteristics were balanced between treatment arms. The median age was 66 years (range, 37-91); 57% of patients were 65 years or older; 60% of patients were male; whites comprised 84% of the study population, Asians 10%, and blacks 4%. The ECOG performance status was 0 in 47%, 1 in 44%, and 2 in 9% of patients, and ISS Stage was I in 43%, II in 32%, and III in 21% of patients. The cytogenetic categories of del 17p and t(4;14) were present in 32% and 9% of patients, respectively. The median number of prior therapies was 2. Thirty-five percent (35%) of patients were refractory (progression during or within 60 days of last therapy) and 65% were relapsed (progression after 60 days of last therapy). Prior therapies included stem cell transplant (55%), bortezomib (70%), melphalan (65%), thalidomide (48%), and lenalidomide (6%).
The efficacy of Elotuzumab was evaluated by progression-free survival (PFS) as assessed by hazard ratio, and overall response rate (ORR) as determined by a blinded Independent Review Committee using the European Group for Blood and Marrow Transplantation (EBMT) response criteria. Efficacy results are shown in Table 7 and Figure 1. The median number of treatment cycles was 19 for the Elotuzumab group and 14 for the comparator arm with a minimum follow-up of two years.
- Table 7: Efficacy Results
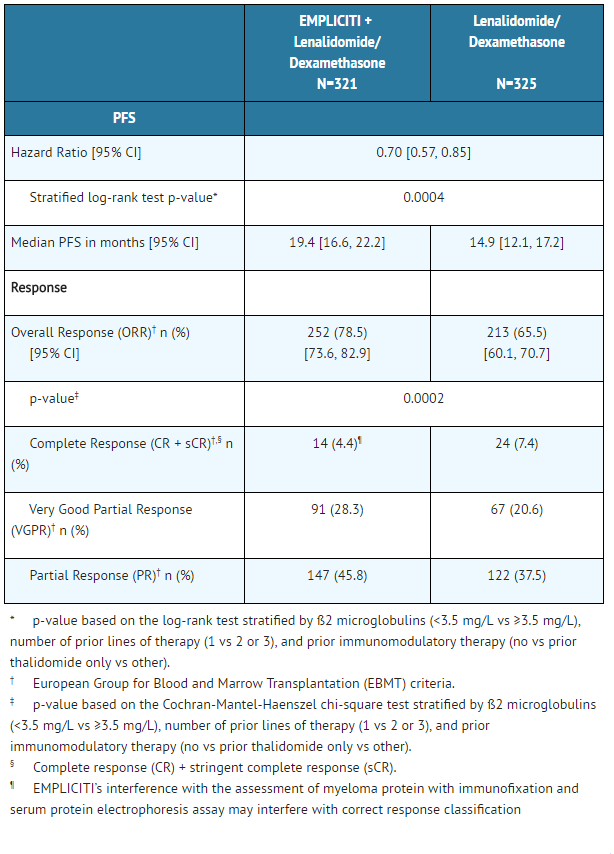
EMPLICITI: Elotuzumab's Brand name
- Figure 1: Progression-Free Survival

EMPLICITI: Elotuzumab's Brand name
The 1- and 2-year rates of PFS for Elotuzumab in combination with lenalidomide and dexamethasone treatment were 68% and 41%, respectively, compared with 57% and 27%, respectively, for lenalidomide and dexamethasone treatment.
At the time of the interim analysis, there were 94 (29%) deaths in the Elotuzumab in combination with lenalidomide and dexamethasone study arm compared to 116 (36%) in the lenalidomide and dexamethasone study arm.
How Supplied
Elotuzumab is white to off-white lyophilized powder available as follows:
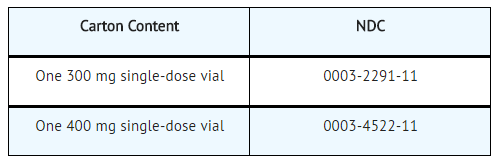
Storage
Store Elotuzumab under refrigeration at 2°C to 8°C (36°F-46°F). Protect Elotuzumab from light by storing in the original package until time of use. Do not freeze or shake.
Images
Drug Images
{{#ask: Page Name::Elotuzumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
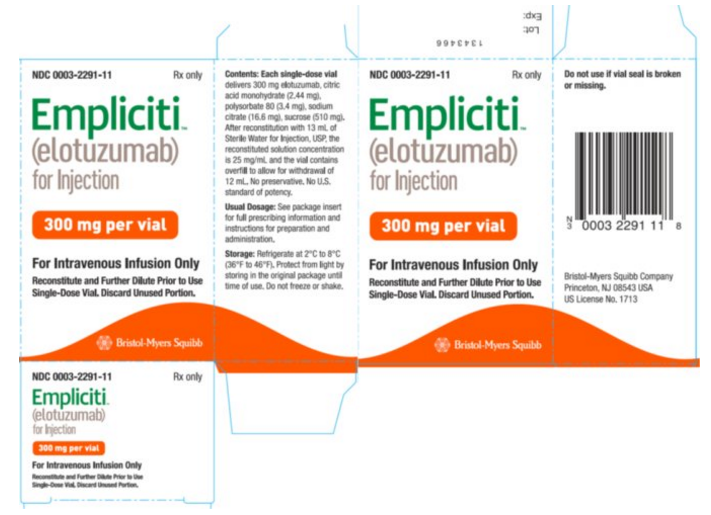
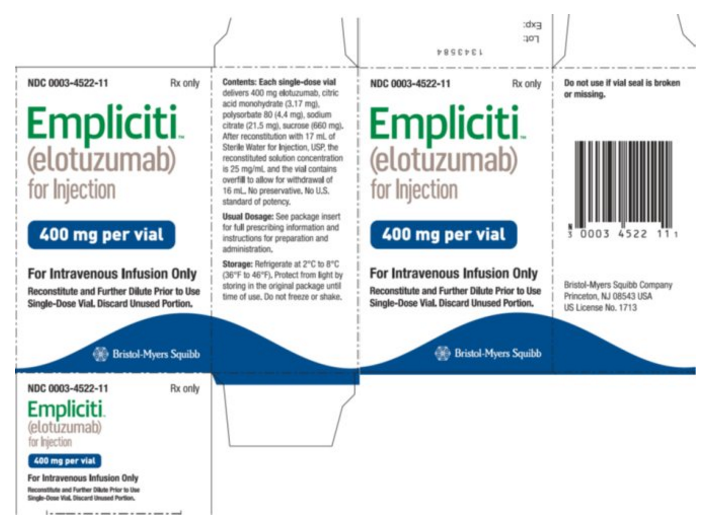
{{#ask: Label Page::Elotuzumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
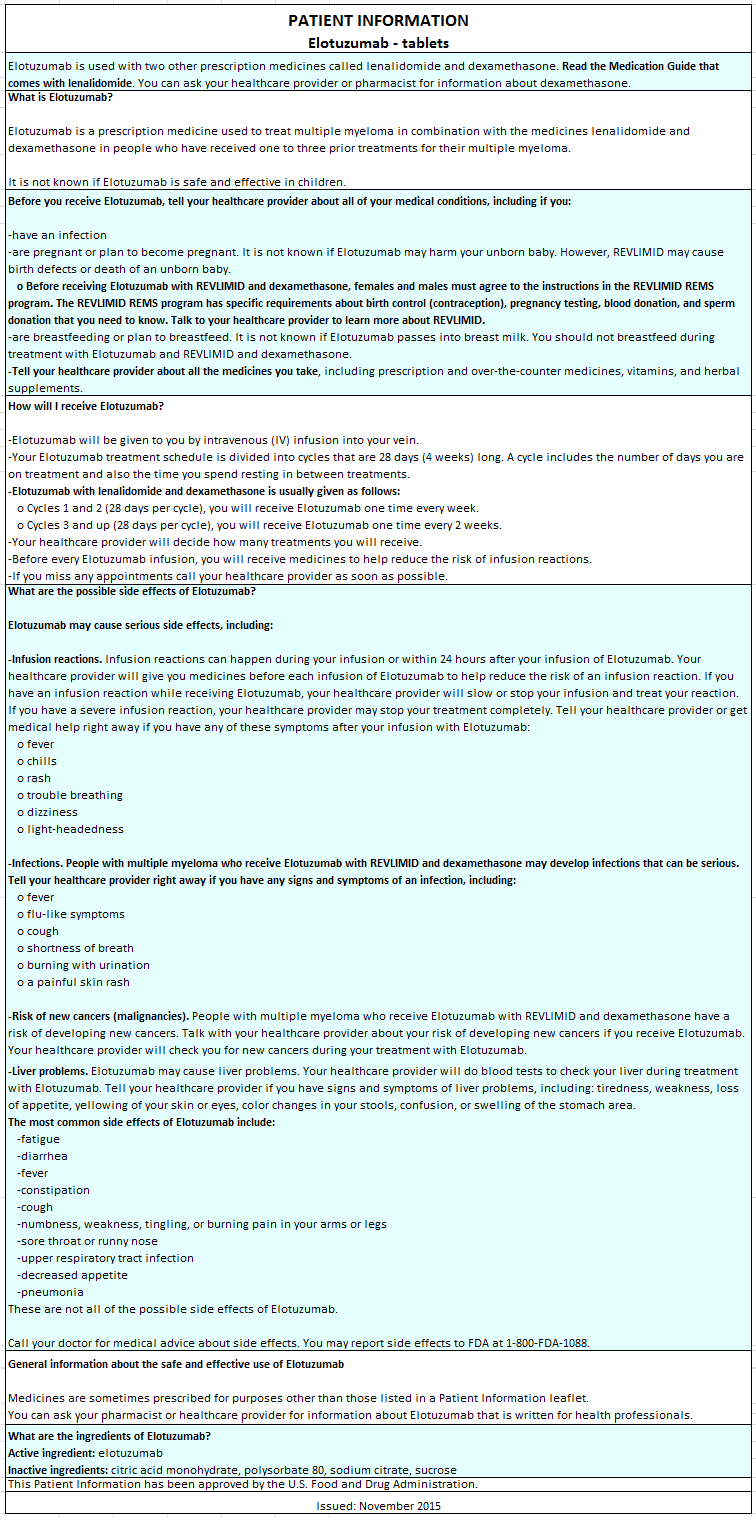
Advise the patient to read the FDA-approved patient labeling (PATIENT INFORMATION).
- Infusion Reactions
- Elotuzumab may cause infusion reactions. Advise patients to contact their healthcare provider if they experience signs and symptoms of infusion reactions, including fever, chills, rash, or breathing problems within 24 hours of infusion.
- Advise patients that they will be required to take the following oral medications prior to Elotuzumab dosing to reduce the risk of infusion reaction:
- - Dexamethasone orally as prescribed
- - H1 blocker: diphenhydramine or equivalent (if oral)
- - H2 blocker: ranitidine or equivalent (if oral)
- - Acetaminophen (650-1000 mg orally)
- Pregnancy
- Advise patients that lenalidomide has the potential to cause fetal harm and has specific requirements regarding contraception, pregnancy testing, blood and sperm donation, and transmission in sperm. Lenalidomide is only available through a REMS program.
- Infections
- Inform patients of the risk of developing infections during treatment with Elotuzumab, and to report any symptoms of infection.
- Second Primary Malignancies
- Inform patients of the risk of developing SPM during treatment with Elotuzumab.
- Inform patients of the risk of hepatotoxicity during treatment with Elotuzumab and to report any signs and symptoms associated with this event to their healthcare provider for evaluation.
Precautions with Alcohol
Alcohol-Elotuzumab interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
EMPLICITI™
Look-Alike Drug Names
There is limited information regarding Elotuzumab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.