Dicyclomine: Difference between revisions
No edit summary |
m (Protected "Dicyclomine": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag= | |authorTag={{VP}}<!--Overview--> | ||
|genericName=Dicyclomine | |||
{ | |aOrAn=an | ||
|drugClass=[[antispasmodic]] and [[anticholinergic]] agent | |||
<!--Overview--> | |indicationType=treatment | ||
|indication=[[functional bowel disorder]] or [[irritable bowel syndrome]] | |||
|genericName= | |adverseReactions=[[dizziness]], [[dry mouth]], [[blurred vision]], [[nausea]], [[somnolence]], [[asthenia]], and [[nervousness]] | ||
Dicyclomine | |||
|aOrAn= | |||
an | |||
|drugClass= | |||
antispasmodic and anticholinergic agent | |||
|indication= | |||
functional bowel | |||
|adverseReactions= | |||
[[dizziness]], [[dry mouth]], vision | |||
<!--Black Box Warning--> | <!--Black Box Warning--> | ||
|blackBoxWarningTitle=Title | |||
|blackBoxWarningTitle= | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
Title | |||
|blackBoxWarningBody= | |||
<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
* Content | * Content | ||
| Line 41: | Line 17: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult======Irritable Bowel Syndrome===== | |||
|fdaLIADAdult= | |||
=====Irritable Bowel Syndrome===== | |||
* Dosing Information | * Dosing Information | ||
| Line 50: | Line 23: | ||
*Oral Dosage and Administration in Adults | *Oral Dosage and Administration in Adults | ||
:*The recommended initial dose is 20 mg four times a day. After one week treatment with the initial dose, the dose may be increased to 40 mg four times a day unless side effects limit dosage escalation. | :*The recommended initial dose is 20 mg four times a day. After one week treatment with the initial dose, the dose may be increased to 40 mg four times a day unless side effects limit dosage escalation. | ||
If efficacy is not achieved within 2 weeks or side effects require doses below 80 mg per day, the drug should be discontinued. Documented safety data are not available for doses above 80 mg daily for periods longer than 2 weeks. | :*If efficacy is not achieved within 2 weeks or side effects require doses below 80 mg per day, the drug should be discontinued. Documented safety data are not available for doses above 80 mg daily for periods longer than 2 weeks. | ||
*Intramuscular Dosage and Administration in Adults | *Intramuscular Dosage and Administration in Adults | ||
:*BENTYL Intramuscular Injection must be administered via intramuscular route only. Do not administer by an other route. The recommended intramuscular dose is 10 mg to 20 mg four times a day. | :*BENTYL Intramuscular Injection must be administered via intramuscular route only. Do not administer by an other route. The recommended intramuscular dose is 10 mg to 20 mg four times a day. | ||
The intramuscular injection is to be used only for 1 or 2 days when the patient cannot take oral medication. | :*The intramuscular injection is to be used only for 1 or 2 days when the patient cannot take oral medication. | ||
:*Intramuscular injection is about twice as bioavailable as oral dosage forms. | :*[[Intramuscular injection]] is about twice as bioavailable as oral dosage forms. | ||
*Preparation for Intramuscular Administration | *Preparation for Intramuscular Administration | ||
:*Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. | :*[[Parenteral]] drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. | ||
:*Aspirate the syringe before injecting to avoid intravascular injection, since thrombosis may occur if the drug is inadvertently injected intravascularly. | :*Aspirate the syringe before injecting to avoid [[intravascular]] injection, since thrombosis may occur if the drug is inadvertently injected [[intravascularly]]. | ||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
<!--Guideline-Supported Use (Adult)--> | <!--Guideline-Supported Use (Adult)--> | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|offLabelAdultGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|offLabelAdultNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | <!--Pediatric Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
|fdaLIADPed= | |||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=*BENTYL is contraindicated in infants less than 6 months of age, nursing mothers], and in patients with: | |||
|contraindications= | :*unstable cardiovascular status in [[acute hemorrhage]] | ||
*BENTYL is contraindicated in infants less than 6 months of age, nursing mothers], and in patients with: | |||
:*unstable cardiovascular status in acute hemorrhage | |||
:*[[myasthenia gravis]] | :*[[myasthenia gravis]] | ||
:*[[glaucoma]] | :*[[glaucoma]] | ||
:*[[obstructive uropathy]] | :*[[obstructive uropathy]] | ||
:*obstructive disease of the gastrointestinal tract | :*[[obstructive disease]] of the [[gastrointestinal tract]] | ||
:*severe [[ulcerative colitis]] | :*severe [[ulcerative colitis]] | ||
:*[[reflux esophagitis]] | :*[[reflux esophagitis]] | ||
<!--Warnings--> | <!--Warnings--> | ||
|warnings=====Precautions==== | |||
|warnings= | |||
====Precautions==== | |||
*Inadvertent Intravenous Administration | *Inadvertent Intravenous Administration | ||
:*BENTYL solution is for intramuscular administration only. Do not administer by any other route. Inadvertent intravenous administration may result in thrombosis, thrombophlebitis and injection site reactions such as pain, edema, skin color change, and reflex sympathetic dystrophy syndrome | :*BENTYL solution is for [[intramuscular]] administration only. Do not administer by any other route. Inadvertent intravenous administration may result in [[thrombosis]], [[thrombophlebitis]] and injection site reactions such as [[pain]], [[edema]], skin color change, and [[reflex sympathetic dystrophy syndrome]]. | ||
*Cardiovascular Conditions | *Cardiovascular Conditions | ||
:*Dicyclomine hydrochloride needs to be used with caution in conditions characterized by tachyarrhythmia such as thyrotoxicosis, congestive heart failure and in cardiac surgery, where they may further accelerate the heart rate. Investigate any tachycardia before administration of dicyclomine hydrochloride. Care is required in patients with coronary heart disease, as ischemia and infarction may be worsened, and in patients with hypertension | :*Dicyclomine hydrochloride needs to be used with caution in conditions characterized by [[tachyarrhythmia]] such as [[thyrotoxicosis]], [[congestive heart failure]] and in [[cardiac surgery]], where they may further accelerate the [[heart rate]]. Investigate any [[tachycardia]] before administration of dicyclomine hydrochloride. Care is required in patients with [[coronary heart disease]], as [[ischemia]] and [[infarction]] may be worsened, and in patients with [[hypertension]]. | ||
*Peripheral and Central Nervous System | *Peripheral and Central Nervous System | ||
:*The peripheral effects of dicyclomine hydrochloride are a consequence of their inhibitory effect on muscarinic receptors of the autonomic nervous system. They include dryness of the mouth with difficulty in swallowing and talking, thirst, reduced bronchial secretions, dilatation of the pupils (mydriasis) with loss of accommodation (cycloplegia) and photophobia, flushing and dryness of the skin, transient bradycardia followed by tachycardia, with palpitations and arrhythmias, and difficulty in micturition, as well as reduction in the tone and motility of the gastrointestinal tract leading to constipation | :*The peripheral effects of dicyclomine hydrochloride are a consequence of their inhibitory effect on [[muscarinic receptors]] of the [[autonomic nervous system]]. They include [[dryness of the mouth]] with difficulty in swallowing and talking, thirst, reduced [[bronchial secretions]], dilatation of the pupils ([[mydriasis]]) with [[loss of accommodation]] ([[cycloplegia]]) and [[photophobia]], [[flushing]] and dryness of the skin, transient [[bradycardia]] followed by tachycardia, with palpitations and [[arrhythmias]], and difficulty in [[micturition]], as well as reduction in the tone and motility of the gastrointestinal tract leading to [[constipation]]. | ||
:*In the presence of high environmental temperature heat prostration can occur with drug use (fever and heat stroke due to decreased sweating). It should also be used cautiously in patients with fever. If symptoms occur, the drug should be discontinued and supportive measures instituted. Because of the inhibitory effect on muscarinic receptors within the autonomic nervous system, caution should be taken in patients with autonomic neuropathy. | :*In the presence of high environmental temperature heat prostration can occur with drug use ([[fever]] and [[heat stroke]] due to decreased [[sweating]]). It should also be used cautiously in patients with fever. If symptoms occur, the drug should be discontinued and supportive measures instituted. Because of the inhibitory effect on [[muscarinic receptors]] within the [[autonomic nervous system]], caution should be taken in patients with [[autonomic neuropathy]]. | ||
:*Central nervous system (CNS) signs and symptoms include confusional state, disorientation, amnesia, hallucinations, dysarthria, ataxia, coma, euphoria, fatigue, insomnia, agitation and mannerisms, and inappropriate affect. | :*Central nervous system (CNS) signs and symptoms include [[confusional state]], disorientation, [[amnesia]], [[hallucinations]], [[dysarthria]], [[ataxia]], coma, [[euphoria]], [[fatigue]], [[insomnia]], [[agitation]] and mannerisms, and inappropriate affect. | ||
:*Psychosis and delirium have been reported in sensitive individuals (such as elderly patients and/or in patients with mental illness) given anticholinergic drugs. These CNS signs and symptoms usually resolve within 12 to 24 hours after discontinuation of the drug. | :*[[Psychosis]] and [[delirium]] have been reported in sensitive individuals (such as elderly patients and/or in patients with mental illness) given [[anticholinergic drugs]]. These CNS signs and symptoms usually resolve within 12 to 24 hours after discontinuation of the drug. | ||
:*BENTYL may produce drowsiness, dizziness or blurred vision. The patient should be warned not to engage in activities requiring mental alertness, such as operating a motor vehicle or other machinery or performing hazardous work while taking BENTYL. | :*BENTYL may produce [[drowsiness]], [[dizziness]] or [[blurred vision]]. The patient should be warned not to engage in activities requiring mental alertness, such as operating a motor vehicle or other machinery or performing hazardous work while taking BENTYL. | ||
*Myasthenia Gravis | *Myasthenia Gravis | ||
:*With overdosage, a curare-like action may occur (i.e., neuromuscular blockade leading to muscular weakness and possible paralysis). It should not be given to patients with myasthenia gravis except to reduce adverse muscarinic effects of an anticholinesterase | :*With overdosage, a [[curare]]-like action may occur (i.e., neuromuscular blockade leading to muscular weakness and possible paralysis). It should not be given to patients with [[myasthenia gravis]] except to reduce adverse muscarinic effects of an [[anticholinesterase]]. | ||
*Intestinal Obstruction | *Intestinal Obstruction | ||
:*Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy. In this instance, treatment with this drug would be inappropriate and possibly harmful | :*Diarrhea may be an early symptom of incomplete [[intestinal obstruction]], especially in patients with [[ileostomy]] or [[colostomy]]. In this instance, treatment with this drug would be inappropriate and possibly harmful. | ||
:*Rarely development of Ogilvie's syndrome (colonic pseudo-obstruction) has been reported. Ogilvie's syndrome is a clinical disorder with signs, symptoms, and radiographic appearance of an acute large bowel obstruction but with no evidence of distal colonic obstruction | :*Rarely development of [[Ogilvie's syndrome]] (colonic pseudo-obstruction) has been reported. [[Ogilvie's syndrome]] is a clinical disorder with signs, symptoms, and radiographic appearance of an acute [[large bowel obstruction]] but with no evidence of distal [[colonic obstruction]]. | ||
*Toxic Dilatation of | *Toxic Dilatation of Intestine megacolon | ||
:*Toxic dilatation of intestine and intestinal perforation is possible when anticholinergic agents are administered in patients with Salmonella dysentery. | :*Toxic dilatation of intestine and [[intestinal perforation]] is possible when [[anticholinergic agents]] are administered in patients with [[Salmonella]] [[dysentery]]. | ||
*Ulcerative Colitis | *Ulcerative Colitis | ||
:*Caution should be taken in patients with ulcerative colitis. Large doses may suppress intestinal motility to the point of producing a paralytic ileus and the use of this drug may precipitate or aggravate the serious complication of toxic megacolon | :*Caution should be taken in patients with [[ulcerative colitis]]. Large doses may suppress intestinal motility to the point of producing a [[paralytic ileus]] and the use of this drug may precipitate or aggravate the serious complication of [[toxic megacolon]]. BENTYL is contraindicated in patients with severe [[ulcerative colitis]]. | ||
*Prostatic Hypertrophy | *Prostatic Hypertrophy | ||
:*BENTYL should be used with caution in patients with known or suspected prostatic enlargement, in whom prostatic enlargement may lead to urinary retention | :*BENTYL should be used with caution in patients with known or suspected [[prostatic enlargement]], in whom [[prostatic]] enlargement may lead to [[urinary retention]]. | ||
*Hepatic and Renal Disease | *Hepatic and Renal Disease | ||
:*BENTYL should be used with caution in patients with known hepatic and renal impairment. | :*BENTYL should be used with caution in patients with known hepatic and [[renal impairment]]. | ||
*Geriatric Population | *Geriatric Population | ||
| Line 206: | Line 106: | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials=*Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |||
*The data described below reflect exposure in controlled clinical trials involving over 100 patients treated for functional bowel/[[irritable bowel syndrome]] with dicyclomine hydrochloride at initial doses of 160 mg daily (40 mg four times a day) | |||
* | *In these trials most of the side effects were typically [[anticholinergic]] in nature and were reported by 61% of the patients. Table 1 presents adverse reactions (MedDRA 13.0 preferred terms) by decreasing order of frequency in a side-by-side comparison with placebo. | ||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*In | *Nine percent (9%) of patients were discontinued from BENTYL because of one or more of these side effects (compared with 2% in the placebo group). In 41% of the patients with side effects, side effects disappeared or were tolerated at the 160 mg daily dose without reduction. A dose reduction from 160 mg daily to an average daily dose of 90 mg was required in 46% of the patients with side effects who then continued to experience a favorable clinical response; their side effects either disappeared or were tolerated. | ||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
|postmarketing=*The following adverse reactions, presented by system organ class in alphabetical order, have been identified during post approval use of BENTYL. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |||
*Cases of [[thrombosis]], [[thrombophlebitis]] and injection site reactions such as local pain, edema, skin color change and even reflex sympathetic [[dystrophy syndrome]] have been reported following inadverent IV injection of BENTYL. | |||
=====Body as a Whole===== | =====Body as a Whole===== | ||
[[Fatigue]], [[malaise]] | |||
=====Cardiovascular===== | =====Cardiovascular===== | ||
[[Palpitations]], [[tachyarrhythmias]] | |||
=====Digestive===== | =====Digestive===== | ||
[[Abdominal distension]], [[abdominal pain]], [[constipation]], [[dry mouth]], [[dyspepsia]], [[nausea]], [[vomiting]] | |||
=====Endocrine===== | =====Endocrine===== | ||
Suppressed [[lactation]] | |||
=====Psychiatric===== | |||
As with the other anti-cholinergic drugs, cases of [[delirium]] or symptoms of [[delirium]] such as [[amnesia]] (or [[transient global amnesia]]), agitation, confusional state, [[delusion]], disorientation, [[hallucination]] (including visual [[hallucination]]) as well as [[mania]], mood altered and [[pseudodementia]], have been reported with the use of Dicyclomine. Nervousness and [[insomnia]] have also been reported. | |||
=====Neurologic===== | =====Neurologic===== | ||
[[Dizziness]], [[headache]], [[somnolence]], [[syncope]] | |||
=====Respiratory===== | =====Respiratory===== | ||
[[Dyspnoea]], nasal congestion | |||
=====Skin and Hypersensitivy Reactions===== | =====Skin and Hypersensitivy Reactions===== | ||
[[Drug hypersensitivity]] including [[face edema]], [[angioedema]], [[anaphylactic shock]], [[dermatitis]] allergic, [[erythema]], [[rash]] | |||
=====Special Senses===== | =====Special Senses===== | ||
[[Cycloplegia]], [[mydriasis]], [[blurred vision]] | |||
<!--Drug Interactions--> | |||
|drugInteractions=*Antiglaucoma Agents | |||
:*Anticholinergics antagonize the effects of [[antiglaucoma]] agents. [[Anticholinergic]] drugs in the presence of increased [[intraocular pressure]] may be hazardous when taken concurrently with agents such as [[corticosteroids]]. Use of BENTYL in patients with glaucoma is not recommended. | |||
*Other Drugs with Anticholinergic Activity | |||
:*The following agents may increase certain actions or side effects of [[anticholinergic]] drugs including BENTYL: [[amantadine]], [[antiarrhythmic agents]] of Class I (e.g., [[quinidine]]), [[antihistamines]], [[antipsychotic agents]] (e.g., [[phenothiazines]]), [[benzodiazepines]], [[MAO inhibitors]], narcotic analgesics (e.g., [[meperidine]]), [[nitrates]] and [[nitrites]], [[sympathomimetic agents]], [[tricyclic antidepressants]], and other drugs having [[anticholinergic activity]]. | |||
*Other Gastrointestinal Motility Drugs | |||
:*Interaction with other gastrointestinal motility drugs may antagonize the effects of drugs that alter gastrointestinal motility, such as [[metoclopramide]]. | |||
*Effect of Antacids | |||
:*Because [[antacids]] may interfere with the absorption of [[anticholinergic agents]] including BENTYL, simultaneous use of these drugs should be avoided. | |||
*Effect on Absorption of Other Drugs | |||
:*Anticholinergic agents may affect [[gastrointestinal absorption]] of various drugs by affecting [[gastrointestinal motility]], such as slowly dissolving dosage forms of [[digoxin]]; increased serum [[digoxin]] concentration may result. | |||
*Effect on Gastric Acid Secretion | |||
:*The inhibiting effects of [[anticholinergic]] drugs on gastric hydrochloric acid secretion are antagonized by agents used to treat [[achlorhydria]] and those used to test gastric secretion. | |||
* | |||
:* | |||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
|useInPregnancyFDA=* '''Pregnancy Category B''' | |||
*Adequate and well-controlled studies have not been conducted with BENTYL in pregnant women at the recommended doses of 80 to 160 mg/day. However, epidemiologic studies did not show an increased risk of structural malformations amoung babies born to women who took products containing dicyclomine hydrochloride at doses up to 40 mg/day during the first trimester of pregnancy. | |||
* | |||
|useInPregnancyAUS= | *Reproduction studies have been performed in rats and rabbits at doses of up to 33 times the maximum recommended human dose based on 160 mg/day (3 mg/kg) and have revealed no evidence of harm to the fetus due to dicyclomine. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. | ||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing=*BENTYL is contraindicated in women who are breastfeeding. Dicyclomine hydrochloride is excreted in human milk. Because of the potential for serious adverse reactions in breast-fed infants from BENTYL, a decision should be made whether to discontine nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | |||
|useInPed=*Safety and effectiveness in pediatric patients have not been established. | |||
*BENTYL is contraindicated in infants less than 6 months of age. There are published cases reporting that the administration of dicyclomine hydrochloride to infants has been followed by serious respiratory symptoms ([[dyspnea]], [[shortness of breath]], breathlessness, respiratory collapse, [[apnea]] and [[asphyxia]]), [[seizures]], [[syncope]], [[pulse rate]] fluctuations, muscular [[hypotonia]], and [[coma]], and death, however; no causal relationship has been established. | |||
|useInGeri=*Clinical studies of BENTYL did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range in adults, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. | |||
There | |||
|useInGeri= | |||
|useInImmunocomp= | *Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. | ||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair=*Effects of renal impairment on PK, safety and efficacy of BENTYL have not been studied. BENTYL drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. BENTYL should be administered with caution in patients with renal impairment. | |||
|useInHepaticImpair=*Effects of renal impairment on PK, safety and efficacy of BENTYL have not been studied. BENTYL should be administered with caution in patients with hepatic impairment. | |||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* Oral | |||
| | * Intramuscular | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | |||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | |||
|overdose====Acute Overdose=== | |||
====Signs and Symptoms==== | |||
*In case of an overdose, patients should contact a physician, poison control centre (1-800-222-1222), or emergency room. | |||
*The signs and symptoms of overdosage include: [[headache]]; [[nausea]]; [[vomiting]]; blurred vision; [[dilated pupils]]; hot, [[dry skin]]; [[dizziness]]; dryness of the mouth; difficulty in [[swallowing]]; and CNS stimulation including convulsion. A curare-like action may occur (i.e., [[neuromuscular blockade]] leading to muscular [[weakness]] and possible [[paralysis]]). | |||
*One reported event included a 37-year-old who reported numbness on the left side, cold fingertips, [[blurred vision]], abdominal and [[flank pain]], [[decreased appetite]], [[dry mouth]], and [[nervousness]] following ingestion of 320 mg daily (four 20 mg tablets four times daily.) These events resolved after discontinuing the dicyclomine. | |||
*The acute oral LD50 of the drug is 625 mg/kg in mice. | |||
*The amount of drug in a single dose that is ordinarily associated with symptoms of overdosage or that is likely to be life-threatening, has not been defined. The maximum human oral dose recorded was 600 mg by mouth in a 10-month-old child and approximately 1500 mg in an adult, each of whom survived. In three of the infants who died following administration of dicyclomine hydrochloride, the blood concentrations of drug were 200, 220, and 505 ng/mL. | |||
*It is not know if BENTYL is [[dialyzable]]. | |||
* | |||
====Management==== | ====Management==== | ||
* | *Treatment should consist of gastric lavage, emetics, and activated charcoal. Sedatives (e.g., short-acting [[barbiturates]], [[benzodiazepines]]) may be used for management of overt signs of excitement. If indicated, an appropriate parenteral [[cholinergic agent]] may be used as an antidote. | ||
===Chronic Overdose=== | ===Chronic Overdose=== | ||
| Line 361: | Line 237: | ||
<!--Drug box 2--> | <!--Drug box 2--> | ||
|drugBox={{Drugbox2 | |||
| Watchedfields = changed | |||
| verifiedrevid = 460784129 | |||
| IUPAC_name = 2-(diethylamino)ethyl 1-cyclohexylcyclohexane-1-carboxylate | |||
| image = Dicyclomine.png | |||
| | <!--Clinical data--> | ||
| tradename = Byclomine, Bentyl, Dibent, Di-Spaz, Dilomine | |||
| Drugs.com = {{drugs.com|international|dicycloverine}} | |||
| MedlinePlus = a684007 | |||
| pregnancy_AU = B1 | |||
| pregnancy_US = B | |||
| legal_AU = S4 | |||
| legal_CA = Rx-only | |||
| legal_UK = POM | |||
| legal_US = Rx-only | |||
<!--Pharmacokinetic data--> | |||
| protein_bound = >99% | |||
| elimination_half-life = 5 h | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 77-19-0 | |||
| ATC_prefix = A03 | |||
| ATC_suffix = AA07 | |||
| PubChem = 3042 | |||
| IUPHAR_ligand = 355 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00804 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 2934 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 4KV4X8IF6V | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D07820 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 4514 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 1123 | |||
<!--Chemical data--> | |||
| C=19 | H=35 | N=1 | O=2 | |||
| molecular_weight = 309.487 g/mol | |||
| smiles = O=C(OCCN(CC)CC)C1(CCCCC1)C2CCCCC2 | |||
| InChI = 1/C19H35NO2/c1-3-20(4-2)15-16-22-18(21)19(13-9-6-10-14-19)17-11-7-5-8-12-17/h17H,3-16H2,1-2H3 | |||
| InChIKey = CURUTKGFNZGFSE-UHFFFAOYAD | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C19H35NO2/c1-3-20(4-2)15-16-22-18(21)19(13-9-6-10-14-19)17-11-7-5-8-12-17/h17H,3-16H2,1-2H3 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = CURUTKGFNZGFSE-UHFFFAOYSA-N | |||
}} | |||
<!--Mechanism of Action--> | <!--Mechanism of Action--> | ||
|mechAction=*Dicyclomine relieves smooth muscle spasm of the gastrointestinal tract. Animal studies indicate that this action is achieved via a dual mechanism: | |||
:*a specific [[anticholinergic]] effect ([[antimuscarinic]]) at the [[acetylcholine-receptor]] sites with approximately 1/8 the milligram potency of atropine (in vitro, guinea pig ileum); and | |||
:*a direct effect upon [[smooth muscle]] (musculotropic) as evidenced by dicyclomine's antagonism of [[bradykinin]]- and [[histamine]]-induced spasms of the isolated guinea pig ileum. | |||
*[[Atropine]] did not affect responses to these two agonists. In vivo studies in cats and dogs showed dicyclomine to be equally potent against [[acetylcholine]] (ACh)- or barium chloride (BaCl2)-induced intestinal spasm while atropine was at least 200 times more potent against effects of ACh than BaCl2. Tests for [[mydriatic]] effects in mice showed that dicyclomine was approximately 1/500 as potent as [[atropine]]; [[antisialagogue]] tests in rabbits showed dicyclomine to be 1/300 as potent as atropine. | |||
* | |||
<!--Structure--> | <!--Structure--> | ||
|structure=* BENTYL is an antispasmodic and anticholinergic (antimuscarinic) agent available in the following dosage forms: | |||
:*BENTYL capsules for oral use contain 10 mg dicyclomine hydrochloride USP. BENTYL 10 mg capsules also contain inactive ingredients: calcium sulfate, corn starch, FD&C Blue No. 1, FD&C Red No. 40, gelatin, lactose, magnesium stearate, pregelatinized corn starch, and titanium dioxide. | |||
:*BENTYL tablets for oral use contain 20 mg dicyclomine hydrochloride USP. BENTYL 20 mg tablets also contain inactive ingredients: acacia, dibasic calcium phosphate, corn starch, FD&C Blue No. 1, lactose, magnesium stearate, pregelatinized corn starch, and sucrose. | |||
:*BENTYL injection is a sterile, pyrogen-free, aqueous solution for intramuscular injection (NOT FOR INTRAVENOUS USE) supplied as an ampoule containing 20 mg/2 mL (10 mg/mL). Each mL contains 10 mg dicyclomine hydrochloride USP in sterile water for injection, made isotonic with sodium chloride. | |||
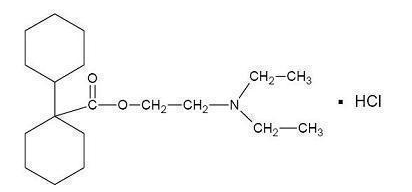
:*BENTYL (dicyclomine hydrochloride) is [bicyclohexyl]-1-carboxylic acid, 2-(diethylamino) ethyl ester, hydrochloride, with a molecular formula of C19H35NO2•HCl and the following structural formula: | |||
| | : [[File:{{PAGENAME}}02.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
*Dicyclomine hydrochloride occurs as a fine, white, crystalline, practically odorless powder with a bitter taste. It is soluble in water, freely soluble in alcohol and chloroform, and very slightly soluble in ether. | |||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
|PD=(BENTYL can inhibit the secretion of saliva and sweat, decrease gastrointestinal secretions and [[motility]], cause [[drowsiness]], [[dilate the pupils]], increase [[heart rate]], and depress [[motor function]]. | |||
|PD= | |||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK=*Absorption and Distribution | |||
:*In man, dicyclomine is rapidly absorbed after oral administration, reaching peak values within 60-90 minutes. Mean volume of distribution for a 20 mg oral dose is approximately 3.65 L/kg suggesting exentsive distribution in tissues. | |||
*Elimination | |||
:*The metabolism of dicyclomine was not studied. The principal route of excretion is via the urine (79.5% of the dose). Excretion also occurs in the feces, but to a lesser extent (8.4%). Mean half-life of plasma elimination in one study was determined to be approximately 1.8 hours when plasma concentrations were measured for 9 hours after a single dose. In subsequent studies, plasma concentrations were followed for up to 24 hours after a single dose, showing a secondary phase of elimination with a somewhat longer half-life. | |||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
|nonClinToxic======Carcinogenesis, Mutagenesis, Impairment of Fertility===== | |||
*Long-term animal studies have not been conducted to evaluate the carcinogenic potential of dicyclomine. In studies in rats at doses of up to 100 mg/kg/day, dicyclomine produced no deleterious effects on breeding, conception, or parturition. | |||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
|clinicalStudies=*In controlled clinical trials involving over 100 patients who received drug, 82% of patients treated for functional bowel/[[irritable bowel syndrome]] with dicyclomine hydrochloride at initial doses of 160 mg daily (40 mg four times daily) demonstrated a favorable clinical response compared with 55% treated with placebo (p<0.05). | |||
| | <!--How Supplied--> | ||
|howSupplied=*BENTYL Capsules | |||
:*10 mg blue capsules, imprinted BENTYL 10, supplied in bottles of 100. Store at room temperature, preferably below 86°F (30°C). | |||
:*NDC number: 58914-012-10. | |||
*BENTYL Tablets | |||
:*20 mg compressed, light blue, round tablets, debossed BENTYL 20, supplied in bottles of 100. To prevent fading, avoid exposure to direct sunlight. Store at room temperature, preferably below 86°F (30°C). | |||
:*NDC 58914-013-10. | |||
*BENTYL Injection | |||
:*20 mg/2 mL (10 mg/mL) injection supplied in boxes of five 20 mg/2 mL ampules (10 mg/mL). Store at room temperature, preferably below 86°F (30°C). Protect from freezing. | |||
:*NDC 58914-080-52. | |||
* | <!--Patient Counseling Information--> | ||
|fdaPatientInfo=*Inadvertent Intravenous Administration | |||
:*BENTYL injection is for intramuscular administration only. Do not administer by any other route. Inadvertent administration may result in [[thrombosis]] or [[thrombophlebitis]] and injection site such as pain, edema, skin color change and even [[reflex sympathetic dystrophy syndrome]]. | |||
*Use in Infants | |||
:*Inform parents and caregivers not to administer BENTYL in infants less than 6 months of age. | |||
*Use in Nursing Mothers | |||
:*Advise lactating women that BENTYL should not be used while breastfeeding their infants . | |||
*Peripheral and Central Nervous System | |||
:*In the presence of a high environmental temperature, heat prostration can occur with BENTYL use ([[fever]] and [[heat stroke]] due to decreased sweating). If symptoms occur, the drug should be discontinued and a physician contacted. BENTYL may produce [[drowsiness]] or blurred vision. The patient should be warned not to engage in activities requiring mental alertness, such as operating a motor vehicle or other machinery or to perform hazardous work while taking BENTYL. | |||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|alcohol= | |||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* BENTYL®<ref>{{Cite web | title = BENTYL - dicyclomine hydrochloride capsule BENTYL - dicyclomine hydrochloride tablet BENTYL - dicyclomine hydrochloride injection, solution | url = http://dailymed.nlm.nih.gov/dailymed/druginfo.cfm?setid=c56598d5-9cd1-4cce-b172-ac338775aec7 }}</ref> | |||
|drugShortage= | |drugShortage= | ||
}} | |||
{{PillImage | |||
|fileName=No image.jpg | |||
}} | }} | ||
{{LabelImage | |||
|fileName={{PAGENAME}}03.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{ | {{LabelImage | ||
|fileName= | |fileName={{PAGENAME}}04.png|This image is provided by the National Library of Medicine. | ||
}} | }} | ||
{{LabelImage | |||
|fileName={{PAGENAME}}05.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}} | |fileName={{PAGENAME}}06.png|This image is provided by the National Library of Medicine. | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}} | |fileName={{PAGENAME}}07.png|This image is provided by the National Library of Medicine. | ||
}} | }} | ||
[[Category:Drug]] | [[Category:Drug]] | ||
[[Category:Anticholinergics]] | |||
Latest revision as of 19:52, 18 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Dicyclomine is an antispasmodic and anticholinergic agent that is FDA approved for the treatment of functional bowel disorder or irritable bowel syndrome. Common adverse reactions include dizziness, dry mouth, blurred vision, nausea, somnolence, asthenia, and nervousness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Irritable Bowel Syndrome
- Dosing Information
- Oral Dosage and Administration in Adults
- The recommended initial dose is 20 mg four times a day. After one week treatment with the initial dose, the dose may be increased to 40 mg four times a day unless side effects limit dosage escalation.
- If efficacy is not achieved within 2 weeks or side effects require doses below 80 mg per day, the drug should be discontinued. Documented safety data are not available for doses above 80 mg daily for periods longer than 2 weeks.
- Intramuscular Dosage and Administration in Adults
- BENTYL Intramuscular Injection must be administered via intramuscular route only. Do not administer by an other route. The recommended intramuscular dose is 10 mg to 20 mg four times a day.
- The intramuscular injection is to be used only for 1 or 2 days when the patient cannot take oral medication.
- Intramuscular injection is about twice as bioavailable as oral dosage forms.
- Preparation for Intramuscular Administration
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Aspirate the syringe before injecting to avoid intravascular injection, since thrombosis may occur if the drug is inadvertently injected intravascularly.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dicyclomine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dicyclomine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Dicyclomine in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dicyclomine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dicyclomine in pediatric patients.
Contraindications
- BENTYL is contraindicated in infants less than 6 months of age, nursing mothers], and in patients with:
- unstable cardiovascular status in acute hemorrhage
- myasthenia gravis
- glaucoma
- obstructive uropathy
- obstructive disease of the gastrointestinal tract
- severe ulcerative colitis
- reflux esophagitis
Warnings
Precautions
- Inadvertent Intravenous Administration
- BENTYL solution is for intramuscular administration only. Do not administer by any other route. Inadvertent intravenous administration may result in thrombosis, thrombophlebitis and injection site reactions such as pain, edema, skin color change, and reflex sympathetic dystrophy syndrome.
- Cardiovascular Conditions
- Dicyclomine hydrochloride needs to be used with caution in conditions characterized by tachyarrhythmia such as thyrotoxicosis, congestive heart failure and in cardiac surgery, where they may further accelerate the heart rate. Investigate any tachycardia before administration of dicyclomine hydrochloride. Care is required in patients with coronary heart disease, as ischemia and infarction may be worsened, and in patients with hypertension.
- Peripheral and Central Nervous System
- The peripheral effects of dicyclomine hydrochloride are a consequence of their inhibitory effect on muscarinic receptors of the autonomic nervous system. They include dryness of the mouth with difficulty in swallowing and talking, thirst, reduced bronchial secretions, dilatation of the pupils (mydriasis) with loss of accommodation (cycloplegia) and photophobia, flushing and dryness of the skin, transient bradycardia followed by tachycardia, with palpitations and arrhythmias, and difficulty in micturition, as well as reduction in the tone and motility of the gastrointestinal tract leading to constipation.
- In the presence of high environmental temperature heat prostration can occur with drug use (fever and heat stroke due to decreased sweating). It should also be used cautiously in patients with fever. If symptoms occur, the drug should be discontinued and supportive measures instituted. Because of the inhibitory effect on muscarinic receptors within the autonomic nervous system, caution should be taken in patients with autonomic neuropathy.
- Central nervous system (CNS) signs and symptoms include confusional state, disorientation, amnesia, hallucinations, dysarthria, ataxia, coma, euphoria, fatigue, insomnia, agitation and mannerisms, and inappropriate affect.
- Psychosis and delirium have been reported in sensitive individuals (such as elderly patients and/or in patients with mental illness) given anticholinergic drugs. These CNS signs and symptoms usually resolve within 12 to 24 hours after discontinuation of the drug.
- BENTYL may produce drowsiness, dizziness or blurred vision. The patient should be warned not to engage in activities requiring mental alertness, such as operating a motor vehicle or other machinery or performing hazardous work while taking BENTYL.
- Myasthenia Gravis
- With overdosage, a curare-like action may occur (i.e., neuromuscular blockade leading to muscular weakness and possible paralysis). It should not be given to patients with myasthenia gravis except to reduce adverse muscarinic effects of an anticholinesterase.
- Intestinal Obstruction
- Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy. In this instance, treatment with this drug would be inappropriate and possibly harmful.
- Rarely development of Ogilvie's syndrome (colonic pseudo-obstruction) has been reported. Ogilvie's syndrome is a clinical disorder with signs, symptoms, and radiographic appearance of an acute large bowel obstruction but with no evidence of distal colonic obstruction.
- Toxic Dilatation of Intestine megacolon
- Toxic dilatation of intestine and intestinal perforation is possible when anticholinergic agents are administered in patients with Salmonella dysentery.
- Ulcerative Colitis
- Caution should be taken in patients with ulcerative colitis. Large doses may suppress intestinal motility to the point of producing a paralytic ileus and the use of this drug may precipitate or aggravate the serious complication of toxic megacolon. BENTYL is contraindicated in patients with severe ulcerative colitis.
- Prostatic Hypertrophy
- BENTYL should be used with caution in patients with known or suspected prostatic enlargement, in whom prostatic enlargement may lead to urinary retention.
- Hepatic and Renal Disease
- BENTYL should be used with caution in patients with known hepatic and renal impairment.
- Geriatric Population
- Dicyclomine hydrochloride should be used with caution in elderly who may be more susceptible to its adverse effects.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The data described below reflect exposure in controlled clinical trials involving over 100 patients treated for functional bowel/irritable bowel syndrome with dicyclomine hydrochloride at initial doses of 160 mg daily (40 mg four times a day)
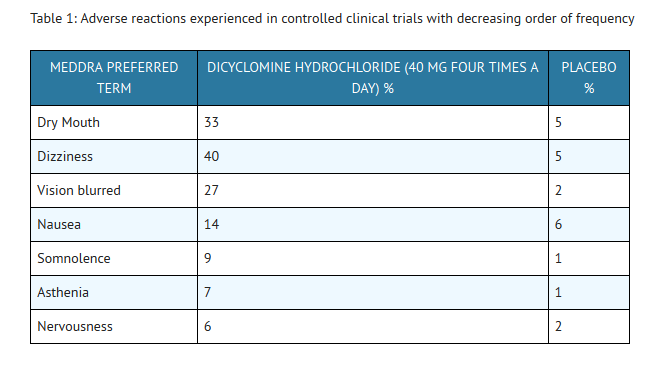
- In these trials most of the side effects were typically anticholinergic in nature and were reported by 61% of the patients. Table 1 presents adverse reactions (MedDRA 13.0 preferred terms) by decreasing order of frequency in a side-by-side comparison with placebo.
- Nine percent (9%) of patients were discontinued from BENTYL because of one or more of these side effects (compared with 2% in the placebo group). In 41% of the patients with side effects, side effects disappeared or were tolerated at the 160 mg daily dose without reduction. A dose reduction from 160 mg daily to an average daily dose of 90 mg was required in 46% of the patients with side effects who then continued to experience a favorable clinical response; their side effects either disappeared or were tolerated.
Postmarketing Experience
- The following adverse reactions, presented by system organ class in alphabetical order, have been identified during post approval use of BENTYL. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Cases of thrombosis, thrombophlebitis and injection site reactions such as local pain, edema, skin color change and even reflex sympathetic dystrophy syndrome have been reported following inadverent IV injection of BENTYL.
Body as a Whole
Cardiovascular
Palpitations, tachyarrhythmias
Digestive
Abdominal distension, abdominal pain, constipation, dry mouth, dyspepsia, nausea, vomiting
Endocrine
Suppressed lactation
Psychiatric
As with the other anti-cholinergic drugs, cases of delirium or symptoms of delirium such as amnesia (or transient global amnesia), agitation, confusional state, delusion, disorientation, hallucination (including visual hallucination) as well as mania, mood altered and pseudodementia, have been reported with the use of Dicyclomine. Nervousness and insomnia have also been reported.
Neurologic
Dizziness, headache, somnolence, syncope
Respiratory
Dyspnoea, nasal congestion
Skin and Hypersensitivy Reactions
Drug hypersensitivity including face edema, angioedema, anaphylactic shock, dermatitis allergic, erythema, rash
Special Senses
Cycloplegia, mydriasis, blurred vision
Drug Interactions
- Antiglaucoma Agents
- Anticholinergics antagonize the effects of antiglaucoma agents. Anticholinergic drugs in the presence of increased intraocular pressure may be hazardous when taken concurrently with agents such as corticosteroids. Use of BENTYL in patients with glaucoma is not recommended.
- Other Drugs with Anticholinergic Activity
- The following agents may increase certain actions or side effects of anticholinergic drugs including BENTYL: amantadine, antiarrhythmic agents of Class I (e.g., quinidine), antihistamines, antipsychotic agents (e.g., phenothiazines), benzodiazepines, MAO inhibitors, narcotic analgesics (e.g., meperidine), nitrates and nitrites, sympathomimetic agents, tricyclic antidepressants, and other drugs having anticholinergic activity.
- Other Gastrointestinal Motility Drugs
- Interaction with other gastrointestinal motility drugs may antagonize the effects of drugs that alter gastrointestinal motility, such as metoclopramide.
- Effect of Antacids
- Because antacids may interfere with the absorption of anticholinergic agents including BENTYL, simultaneous use of these drugs should be avoided.
- Effect on Absorption of Other Drugs
- Anticholinergic agents may affect gastrointestinal absorption of various drugs by affecting gastrointestinal motility, such as slowly dissolving dosage forms of digoxin; increased serum digoxin concentration may result.
- Effect on Gastric Acid Secretion
- The inhibiting effects of anticholinergic drugs on gastric hydrochloric acid secretion are antagonized by agents used to treat achlorhydria and those used to test gastric secretion.
Use in Specific Populations
Pregnancy
- Pregnancy Category B
- Adequate and well-controlled studies have not been conducted with BENTYL in pregnant women at the recommended doses of 80 to 160 mg/day. However, epidemiologic studies did not show an increased risk of structural malformations amoung babies born to women who took products containing dicyclomine hydrochloride at doses up to 40 mg/day during the first trimester of pregnancy.
- Reproduction studies have been performed in rats and rabbits at doses of up to 33 times the maximum recommended human dose based on 160 mg/day (3 mg/kg) and have revealed no evidence of harm to the fetus due to dicyclomine. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dicyclomine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Dicyclomine during labor and delivery.
Nursing Mothers
- BENTYL is contraindicated in women who are breastfeeding. Dicyclomine hydrochloride is excreted in human milk. Because of the potential for serious adverse reactions in breast-fed infants from BENTYL, a decision should be made whether to discontine nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
- BENTYL is contraindicated in infants less than 6 months of age. There are published cases reporting that the administration of dicyclomine hydrochloride to infants has been followed by serious respiratory symptoms (dyspnea, shortness of breath, breathlessness, respiratory collapse, apnea and asphyxia), seizures, syncope, pulse rate fluctuations, muscular hypotonia, and coma, and death, however; no causal relationship has been established.
Geriatic Use
- Clinical studies of BENTYL did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range in adults, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
- Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Dicyclomine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Dicyclomine with respect to specific racial populations.
Renal Impairment
- Effects of renal impairment on PK, safety and efficacy of BENTYL have not been studied. BENTYL drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. BENTYL should be administered with caution in patients with renal impairment.
Hepatic Impairment
- Effects of renal impairment on PK, safety and efficacy of BENTYL have not been studied. BENTYL should be administered with caution in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Dicyclomine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Dicyclomine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intramuscular
Monitoring
There is limited information regarding Monitoring of Dicyclomine in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Dicyclomine in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- In case of an overdose, patients should contact a physician, poison control centre (1-800-222-1222), or emergency room.
- The signs and symptoms of overdosage include: headache; nausea; vomiting; blurred vision; dilated pupils; hot, dry skin; dizziness; dryness of the mouth; difficulty in swallowing; and CNS stimulation including convulsion. A curare-like action may occur (i.e., neuromuscular blockade leading to muscular weakness and possible paralysis).
- One reported event included a 37-year-old who reported numbness on the left side, cold fingertips, blurred vision, abdominal and flank pain, decreased appetite, dry mouth, and nervousness following ingestion of 320 mg daily (four 20 mg tablets four times daily.) These events resolved after discontinuing the dicyclomine.
- The acute oral LD50 of the drug is 625 mg/kg in mice.
- The amount of drug in a single dose that is ordinarily associated with symptoms of overdosage or that is likely to be life-threatening, has not been defined. The maximum human oral dose recorded was 600 mg by mouth in a 10-month-old child and approximately 1500 mg in an adult, each of whom survived. In three of the infants who died following administration of dicyclomine hydrochloride, the blood concentrations of drug were 200, 220, and 505 ng/mL.
- It is not know if BENTYL is dialyzable.
Management
- Treatment should consist of gastric lavage, emetics, and activated charcoal. Sedatives (e.g., short-acting barbiturates, benzodiazepines) may be used for management of overt signs of excitement. If indicated, an appropriate parenteral cholinergic agent may be used as an antidote.
Chronic Overdose
There is limited information regarding Chronic Overdose of Dicyclomine in the drug label.
Pharmacology
| |
Dicyclomine
| |
| Systematic (IUPAC) name | |
| 2-(diethylamino)ethyl 1-cyclohexylcyclohexane-1-carboxylate | |
| Identifiers | |
| CAS number | |
| ATC code | A03 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 309.487 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | >99% |
| Metabolism | ? |
| Half life | 5 h |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
Prescription Only (S4)(AU) ?(CA) POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
- Dicyclomine relieves smooth muscle spasm of the gastrointestinal tract. Animal studies indicate that this action is achieved via a dual mechanism:
- a specific anticholinergic effect (antimuscarinic) at the acetylcholine-receptor sites with approximately 1/8 the milligram potency of atropine (in vitro, guinea pig ileum); and
- a direct effect upon smooth muscle (musculotropic) as evidenced by dicyclomine's antagonism of bradykinin- and histamine-induced spasms of the isolated guinea pig ileum.
- Atropine did not affect responses to these two agonists. In vivo studies in cats and dogs showed dicyclomine to be equally potent against acetylcholine (ACh)- or barium chloride (BaCl2)-induced intestinal spasm while atropine was at least 200 times more potent against effects of ACh than BaCl2. Tests for mydriatic effects in mice showed that dicyclomine was approximately 1/500 as potent as atropine; antisialagogue tests in rabbits showed dicyclomine to be 1/300 as potent as atropine.
Structure
- BENTYL is an antispasmodic and anticholinergic (antimuscarinic) agent available in the following dosage forms:
- BENTYL capsules for oral use contain 10 mg dicyclomine hydrochloride USP. BENTYL 10 mg capsules also contain inactive ingredients: calcium sulfate, corn starch, FD&C Blue No. 1, FD&C Red No. 40, gelatin, lactose, magnesium stearate, pregelatinized corn starch, and titanium dioxide.
- BENTYL tablets for oral use contain 20 mg dicyclomine hydrochloride USP. BENTYL 20 mg tablets also contain inactive ingredients: acacia, dibasic calcium phosphate, corn starch, FD&C Blue No. 1, lactose, magnesium stearate, pregelatinized corn starch, and sucrose.
- BENTYL injection is a sterile, pyrogen-free, aqueous solution for intramuscular injection (NOT FOR INTRAVENOUS USE) supplied as an ampoule containing 20 mg/2 mL (10 mg/mL). Each mL contains 10 mg dicyclomine hydrochloride USP in sterile water for injection, made isotonic with sodium chloride.
- BENTYL (dicyclomine hydrochloride) is [bicyclohexyl]-1-carboxylic acid, 2-(diethylamino) ethyl ester, hydrochloride, with a molecular formula of C19H35NO2•HCl and the following structural formula:
- Dicyclomine hydrochloride occurs as a fine, white, crystalline, practically odorless powder with a bitter taste. It is soluble in water, freely soluble in alcohol and chloroform, and very slightly soluble in ether.
Pharmacodynamics
(BENTYL can inhibit the secretion of saliva and sweat, decrease gastrointestinal secretions and motility, cause drowsiness, dilate the pupils, increase heart rate, and depress motor function.
Pharmacokinetics
- Absorption and Distribution
- In man, dicyclomine is rapidly absorbed after oral administration, reaching peak values within 60-90 minutes. Mean volume of distribution for a 20 mg oral dose is approximately 3.65 L/kg suggesting exentsive distribution in tissues.
- Elimination
- The metabolism of dicyclomine was not studied. The principal route of excretion is via the urine (79.5% of the dose). Excretion also occurs in the feces, but to a lesser extent (8.4%). Mean half-life of plasma elimination in one study was determined to be approximately 1.8 hours when plasma concentrations were measured for 9 hours after a single dose. In subsequent studies, plasma concentrations were followed for up to 24 hours after a single dose, showing a secondary phase of elimination with a somewhat longer half-life.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term animal studies have not been conducted to evaluate the carcinogenic potential of dicyclomine. In studies in rats at doses of up to 100 mg/kg/day, dicyclomine produced no deleterious effects on breeding, conception, or parturition.
Clinical Studies
- In controlled clinical trials involving over 100 patients who received drug, 82% of patients treated for functional bowel/irritable bowel syndrome with dicyclomine hydrochloride at initial doses of 160 mg daily (40 mg four times daily) demonstrated a favorable clinical response compared with 55% treated with placebo (p<0.05).
How Supplied
- BENTYL Capsules
- 10 mg blue capsules, imprinted BENTYL 10, supplied in bottles of 100. Store at room temperature, preferably below 86°F (30°C).
- NDC number: 58914-012-10.
- BENTYL Tablets
- 20 mg compressed, light blue, round tablets, debossed BENTYL 20, supplied in bottles of 100. To prevent fading, avoid exposure to direct sunlight. Store at room temperature, preferably below 86°F (30°C).
- NDC 58914-013-10.
- BENTYL Injection
- 20 mg/2 mL (10 mg/mL) injection supplied in boxes of five 20 mg/2 mL ampules (10 mg/mL). Store at room temperature, preferably below 86°F (30°C). Protect from freezing.
- NDC 58914-080-52.
Storage
There is limited information regarding Dicyclomine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Dicyclomine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Dicyclomine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Inadvertent Intravenous Administration
- BENTYL injection is for intramuscular administration only. Do not administer by any other route. Inadvertent administration may result in thrombosis or thrombophlebitis and injection site such as pain, edema, skin color change and even reflex sympathetic dystrophy syndrome.
- Use in Infants
- Inform parents and caregivers not to administer BENTYL in infants less than 6 months of age.
- Use in Nursing Mothers
- Advise lactating women that BENTYL should not be used while breastfeeding their infants .
- Peripheral and Central Nervous System
- In the presence of a high environmental temperature, heat prostration can occur with BENTYL use (fever and heat stroke due to decreased sweating). If symptoms occur, the drug should be discontinued and a physician contacted. BENTYL may produce drowsiness or blurred vision. The patient should be warned not to engage in activities requiring mental alertness, such as operating a motor vehicle or other machinery or to perform hazardous work while taking BENTYL.
Precautions with Alcohol
- Alcohol-Dicyclomine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- BENTYL®[1]
Look-Alike Drug Names
There is limited information regarding Dicyclomine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Dicyclomine
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Dicyclomine |Label Name=Dicyclomine03.png
}}
{{#subobject:
|Label Page=Dicyclomine |Label Name=Dicyclomine04.png
}}
{{#subobject:
|Label Page=Dicyclomine |Label Name=Dicyclomine05.png
}}
{{#subobject:
|Label Page=Dicyclomine |Label Name=Dicyclomine06.png
}}
{{#subobject:
|Label Page=Dicyclomine |Label Name=Dicyclomine07.png
}}