Cidofovir
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning
See full prescribing information for complete Boxed Warning.
Condition Name: Renal impairment is the major toxicity of vistide. Cases of acute renal failure resulting in dialysis and/or contributing to death have occurred with as few as one or two doses of vistide. To reduce possible nephrotoxicity, intravenous prehydration with normal saline and administration of probenecid must be used with each vistide infusion. Renal function (serum creatinine and urine protein) must be monitored within 48 hours prior to each dose of vistide and the dose of vistide modified for changes in renal function as appropriate. vistide is contraindicated in patients who are receiving other nephrotoxic agents.
Neutropenia has been observed in association with vistide treatment. Therefore, neutrophil counts should be monitored during visited therapy. Vistide is indicated only for the treatment of CMV retinitis in patients with acquired immunodeficiency syndrome. In animal studies cidofovir was carcinogenic, teratogenic and caused hypospermia.
|
Overview
Cidofovir is an antiviral, cytosine nucleoside analog that is FDA approved for the treatment of Citomegalovirus (CMV) retinitis in patients with acquired immunodeficiency syndrome (AIDS). The safety and efficacy of Cidofovir have not been established for treatment of other CMV infections (such as pneumonitis or gastroenteritis), congenital or neonatal CMV disease, or CMV disease in non-HIV-infected individuals.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Cidofovir FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cidofovir in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cidofovir in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Cidofovir FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cidofovir in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cidofovir in pediatric patients.
Contraindications
- Initiation of therapy with VISTIDE is contraindicated in patients with a serum creatinine > 1.5 mg/dL, a calculated creatinine clearance ≤ 55 mL/min, or a urine protein ≥ 100 mg/dL (equivalent to ≥ 2+ proteinuria).
- VISTIDE is contraindicated in patients receiving agents with nephrotoxic potential. Such agents must be discontinued at least seven days prior to starting therapy with VISTIDE.
- VISTIDE is contraindicated in patients with hypersensitivity to cidofovir.
- VISTIDE is contraindicated in patients with a history of clinically severe hypersensitivity to probenecid or other sulfa-containing medications.
- Direct intraocular injection of VISTIDE is contraindicated; direct injection of cidofovir has been associated with iritis, ocular hypotony, and permanent impairment of vision.
Warnings
|
Warning
See full prescribing information for complete Boxed Warning.
Condition Name: Renal impairment is the major toxicity of vistide. Cases of acute renal failure resulting in dialysis and/or contributing to death have occurred with as few as one or two doses of vistide. To reduce possible nephrotoxicity, intravenous prehydration with normal saline and administration of probenecid must be used with each vistide infusion. Renal function (serum creatinine and urine protein) must be monitored within 48 hours prior to each dose of vistide and the dose of vistide modified for changes in renal function as appropriate. vistide is contraindicated in patients who are receiving other nephrotoxic agents.
Neutropenia has been observed in association with vistide treatment. Therefore, neutrophil counts should be monitored during visited therapy. Vistide is indicated only for the treatment of CMV retinitis in patients with acquired immunodeficiency syndrome. In animal studies cidofovir was carcinogenic, teratogenic and caused hypospermia.
|
Nephrotoxicity
Dose-dependent nephrotoxicity is the major dose-limiting toxicity related to VISTIDE administration. Cases of acute renal failure resulting in dialysis and/or contributing to death have occurred with as few as one or two doses of VISTIDE. Renal function (serum creatinine and urine protein) must be monitored within 48 hours prior to each dose of VISTIDE. Dose adjustment or discontinuation is required for changes in renal function (serum creatinine and/or urine protein) while on therapy. Proteinuria, as measured by urinalysis in a clinical laboratory, may be an early indicator of VISTIDE-related nephrotoxicity. Continued administration of VISTIDE may lead to additional proximal tubule cell injury, which may result in glycosuria, decreases in serum phosphate, uric acid, and bicarbonate, elevations in serum creatinine, and/or acute renal failure, in some cases, resulting in the need for dialysis. Patients with these adverse events occurring concurrently and meeting a criteria of Fanconi's syndrome have been reported. Renal function that did not return to baseline after drug discontinuation has been observed in clinical studies of VISTIDE.
Intravenous normal saline hydration and oral probenecid must accompany each VISTIDE infusion. Probenecid is known to interact with the metabolism or renal tubular excretion of many drugs. The safety of VISTIDE has not been evaluated in patients receiving other known potentially nephrotoxic agents, such as intravenous aminoglycosides (tobramycin, gentamicin, and amikacin), amphotericin B, foscarnet, intravenous pentamidine, vancomycin, and non-steroidal anti-inflammatory agents.
Preexisting Renal Impairment
Initiation of therapy with VISTIDE is contraindicated in patients with a baseline serum creatinine > 1.5 mg/dL, a creatinine clearance ≤ 55 mL/min, or a urine protein ≥ 100 mg/dL (equivalent to ≥ 2+ proteinuria).
Hematological Toxicity
Neutropenia may occur during VISTIDE therapy. Neutrophil count should be monitored while receiving VISTIDE therapy.
Decreased Intraocular Pressure/Ocular Hypotony
Decreased intraocular pressure may occur during VISTIDE therapy, and in some instances has been associated with decreased visual acuity. Intraocular pressure should be monitored during VISTIDE therapy.
Metabolic Acidosis
Decreased serum bicarbonate associated with proximal tubule injury and renal wasting syndrome (including Fanconi's syndrome) have been reported in patients receiving VISTIDE. Cases of metabolic acidosis in association with liver dysfunction and pancreatitis resulting in death have been reported in patients receiving VISTIDE.
Adverse Reactions
Clinical Trials Experience
Nephrotoxicity
Renal toxicity, as manifested by ≥ 2+ proteinuria, serum creatinine elevations of ≥ 0.4 mg/dL, or decreased creatinine clearance ≤ 55 mL/min, occurred in 79 of 135 (59%) patients receiving VISTIDE at a maintenance dose of 5 mg/kg every other week. Maintenance dose reductions from 5 mg/kg to 3 mg/kg due to proteinuria or serum creatinine elevations were made in 12 of 41 (29%) patients who had not received prior therapy for CMV retinitis (Study 106) and in 19 of 74 (26%) patients who had received prior therapy for CMV retinitis (Study 107). Prior foscarnet use has been associated with an increased risk of nephrotoxicity; therefore, such patients must be monitored closely.
Neutropenia
In clinical trials, at the 5 mg/kg maintenance dose, a decrease in absolute neutrophil count to ≤ 500 cells/mm 3 occurred in 24% of patients. Granulocyte colony stimulating factor (GCSF) was used in 39% of patients.
Decreased Intraocular Pressure/Ocular Hypotony
Among the subset of patients monitored for intraocular pressure changes, a ≥ 50% decrease from baseline intraocular pressure was reported in 17 of 70 (24%) patients at the 5 mg/kg maintenance dose. Severe hypotony (intraocular pressure of 0–1 mm Hg) has been reported in 3 patients. Risk of ocular hypotony may be increased in patients with preexisting diabetes mellitus.
Anterior Uveitis/Iritis
Uveitis or iritis has been reported in clinical trials and during postmarketing in patients receiving VISTIDE therapy. Uveitis or iritis was reported in 15 of 135 (11%) patients receiving 5 mg/kg maintenance dosing. Treatment with topical corticosteroids with or without topical cycloplegic agents may be considered. Patients should be monitored for signs and symptoms of uveitis/iritis during VISTIDE therapy.
Metabolic Acidosis
A diagnosis of Fanconi's syndrome, as manifested by multiple abnormalities of proximal renal tubular function, was reported in 1% of patients. Decreases in serum bicarbonate to ≤ 16 mEq/L occurred in 16% of cidofovir-treated patients. Cases of metabolic acidosis in association with liver dysfunction and pancreatitis resulting in death have been reported in patients receiving VISTIDE. In clinical trials, VISTIDE was withdrawn due to adverse events in 39% of patients treated with 5 mg/kg every other week as maintenance therapy.
The incidence of adverse reactions reported as serious in three controlled clinical studies in patients with CMV retinitis, regardless of presumed relationship to drug, is listed in Table 4.
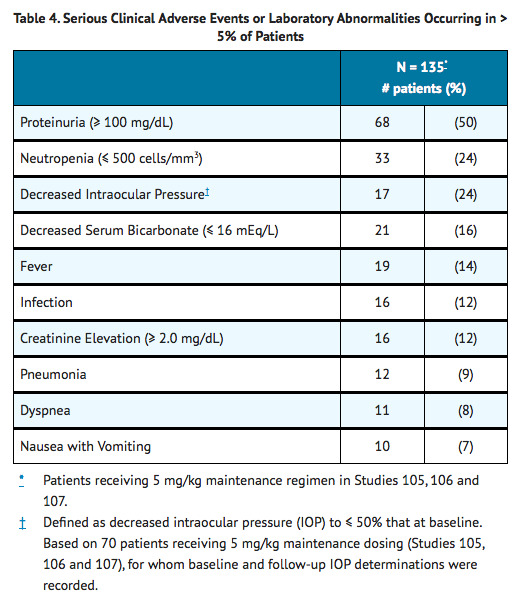
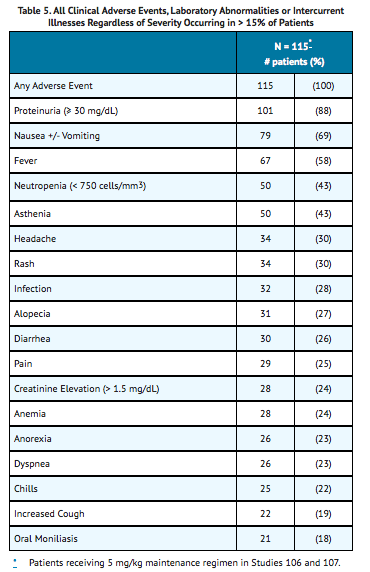
The most frequently reported adverse events regardless of relationship to study drugs (cidofovir or probenecid) or severity are shown in Table 5.
The following additional list of adverse events/intercurrent illnesses have been observed in clinical studies of VISTIDE and are listed below regardless of causal relationship to VISTIDE. Evaluation of these reports was difficult because of the diverse manifestations of the underlying disease and because most patients received numerous concomitant medicines.
Body as a Whole
- Abdominal pain
- Accidental injury
- AIDS
- Allergic reaction
- Back pain
- Catheter blocked
- Cellulitis
- Chest pain
- Chills and fever
- Cryptococcosis
- Cyst
- Death
- Face edema
- Flu-like syndrome
- Hypothermia
- Injection site reaction
- Malaise
- Mucous membrane disorder
- Neck pain
- Overdose
- Photosensitivity reaction
- Sarcoma
- Sepsis
Cardiovascular System
- Cardiomyopathy
- Cardiovascular disorder
- Congestive heart failure
- Hypertension
- hypotension
- Migraine
- Pallor
- Peripheral vascular disorder
- Phlebitis
- Postural hypotension
- Shock
- Syncope
- Tachycardia
- Vascular disorder
- Edema
Digestive System
- Cholangitis
- Colitis
- Constipation
- Esophagitis
- Dyspepsia
- Dysphagia
- Fecal incontinence
- Flatulence
- Gastritis
- Gastrointestinal hemorrhage
- Gingivitis
- Hepatitis
- Hepatomegaly
- Hepatosplenomegaly
- Jaundice
- Abnormal liver function
- Liver damage
- Liver necrosis
- Melena
- Pancreatitis
- Proctitis
- Rectal disorder
- Stomatitis
- Aphthous stomatitis
- Tongue discoloration
- Mouth ulceration
- Tooth caries
Endocrine System
Hemic & Lymphatic System
- Hypochromic anemia
- Leukocytosis
- Leukopenia
- Lymphadenopathy
- Lymphoma like reaction
- Pancytopenia
- Splenic disorder
- Splenomegaly
- Thrombocytopenia
- Thrombocytopenic purpura
Metabolic & Nutritional System
- Cachexia
- Dehydration
- Edema
- Hypercalcemia
- Hyperglycemia
- Hyperkalemia
- Hyperlipemia
- Hypocalcemia
- Hypoglycemia
- Hypoglycemic reaction
- Hypokalemia
- Hypomagnesemia
- Hyponatremia
- Hypophosphatemia
- Hypoproteinemia
- Increased alkaline phosphatase
- Increased BUN
- Increased lactic dehydrogenase
- Increased SGOT/SGPT
- Peripheral edema
- Respirator alkalosis
- Thirst
- Weight loss
- Weight gain
Musculoskeletal System
- Arthralgia
- Arthrosis
- Bone necrosis
- Bone pain
- Joint disorder
- Leg cramps
- Myalgia
- Myasthenia
- Pathological fracture
Nervous System
- Abnormal dreams
- Abnormal gait
- Acute brain syndrome
- Agitation
- Amnesia
- Anxiety
- Ataxia
- Cerebrovascular disorder
- Confusion
- Convulsion
- Delirium
- Dementia
- Depression
- Dizziness
- Drug dependence
- Dry mouth
- Encephalopathy
- Facial paralysis
- Hallucinations
- Hemiplegia
- Hyperesthesia
- Hypertonia
- Hypotony
- Incoordination
- Increased libido
- Insomnia
- Myoclonus
- Nervousness
- Neuropathy
- Paresthesia
- Personality disorder
- Somnolence
- Speech disorder
- Tremor
- Twitching
- Vasodilatation
- Vertigo
Respiratory System
- Asthma
- Bronchitis
- Epistaxis
- Hemoptysis
- Hiccups
- Hyperventilation
- Hjypoxia
- Increased sputum
- Larynx edema
- Lung disorder
- Pharyngitis
- Pneumothorax
- Rhinitis
- Sinusitis
Skin & Appendages
- Acne
- Angioedema
- Dry skin
- Eczema
- Exfoliative dermatitis
- Furunculosis
- Herpes simplex
- Nail disorder
- Pruritus
- Rash
- Seborrhea
- Skin discoloration
- Skin disorder
- Skin hypertrophy
- Skin ulcer
- Sweating
- Urticaria
Special Senses
- Abnormal vision
- Amblyopia
- Blindness
- Cataract
- Conjunctivitis
- Corneal lesion
- Corneal opacity
- Diplopia
- Dry eyes
- Ear disorder
- Ear pain
- Eye disorder
- Eye pain
- Hyperacusis
- Iritis
- Keratitis
- Miosis
- Otitis externa
- Otitis media
- Refraction disorder
- Retinal detachment
- Retinal disorder
- Taste perversion
- Tinnitus
- Uveitis
- Visual field defect
- Hearing loss
Urogenital System
- Decreased creatinine clearance
- Dysuria
- Glycosuria
- Hematuria
- Kidney stone
- Mastitis
- Metorrhagia
- Nocturia
- Polyuria
- Prostatic disorder
- Toxic nephrophathy
- Urethritis
- Urinary casts
- Urinary incontinence
- Urinary retention
- Urinary tract infection
Postmarketing Experience
There is limited information regarding Cidofovir Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Cidofovir Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Cidofovir was embryotoxic (reduced fetal body weights) in rats at 1.5 mg/kg/day and in rabbits at 1.0 mg/kg/day, doses which were also maternally toxic, following daily intravenous dosing during the period of organogenesis. The no-observable-effect levels for embryotoxicity in rats (0.5 mg/kg/day) and in rabbits (0.25 mg/kg/day) were approximately 0.04 and 0.05 times the clinical dose (5 mg/kg every other week) based on AUC, respectively. An increased incidence of fetal external, soft tissue and skeletal anomalies (meningocele, short snout, and short maxillary bones) occurred in rabbits at the high dose (1.0 mg/kg/day) which was also maternally toxic. There are no adequate and well-controlled studies in pregnant women. VISTIDE should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cidofovir in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Cidofovir during labor and delivery.
Nursing Mothers
It is not known whether cidofovir is excreted in human milk. Since many drugs are excreted in human milk and because of the potential for adverse reactions as well as the potential for tumorigenicity shown for cidofovir in animal studies, VISTIDE should not be administered to nursing mothers. The U.S. Public Health Service Centers for Disease Control and Prevention advises HIV-infected women not to breast-feed to avoid postnatal transmission of HIV to a child who may not yet be infected.
Pediatric Use
Safety and effectiveness in children have not been studied. The use of VISTIDE in children with AIDS warrants extreme caution due to the risk of long-term carcinogenicity and reproductive toxicity. Administration of VISTIDE to children should be undertaken only after careful evaluation and only if the potential benefits of treatment outweigh the risks.
Geriatic Use
No studies of the safety or efficacy of VISTIDE in patients over the age of 60 have been conducted. Since elderly individuals frequently have reduced glomerular filtration, particular attention should be paid to assessing renal function before and during VISTIDE administration
Gender
There is no FDA guidance on the use of Cidofovir with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cidofovir with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Cidofovir in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Cidofovir in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Cidofovir in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Cidofovir in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Cidofovir Administration in the drug label.
Monitoring
There is limited information regarding Cidofovir Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Cidofovir and IV administrations.
Overdosage
Two cases of cidofovir overdose have been reported. These patients received single doses of VISTIDE at 16.3 mg/kg and 17.4 mg/kg, respectively, with concomitant oral probenecid and intravenous hydration. In both cases, the patients were hospitalized and received oral probenecid (one gram three times daily) and vigorous intravenous hydration with normal saline for 3 to 5 days. Significant changes in renal function were not observed in either patient.
Pharmacology
Mechanism of Action
Cidofovir suppresses cytomegalovirus (CMV) replication by selective inhibition of viral DNA synthesis. Biochemical data support selective inhibition of CMV DNA polymerase by cidofovir diphosphate, the active intracellular metabolite of cidofovir. Cidofovir diphosphate inhibits herpesvirus polymerases at concentrations that are 8- to 600-fold lower than those needed to inhibit human cellular DNA polymerases alpha, beta, and gamma1, 2, 3. Incorporation of cidofovir into the growing viral DNA chain results in reductions in the rate of viral DNA synthesis.
Structure
The chemical name of cidofovir is 1-[(S)-3-hydroxy-2-(phosphonomethoxy)propyl]cytosine dihydrate (HPMPC), with the molecular formula of C8H14N3O6P•2H2O and a molecular weight of 315.22 (279.19 for anhydrous). The chemical structure is:

Pharmacodynamics
There is limited information regarding Cidofovir Pharmacodynamics in the drug label.
Pharmacokinetics
VISTIDE must be administered with probenecid. The pharmacokinetics of cidofovir, administered both without and with probenecid, are described below.
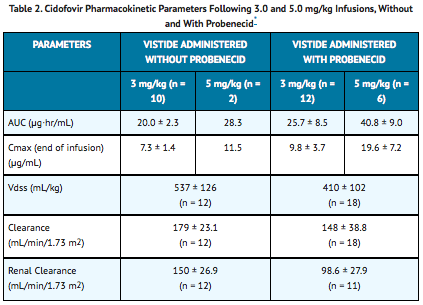
The pharmacokinetics of cidofovir without probenecid were evaluated in 27 HIV-infected patients with or without asymptomatic CMV infection. Dose-independent pharmacokinetics were demonstrated after one hr infusions of 1.0 (n = 5), 3.0 (n = 10), 5.0 (n = 2) and 10.0 (n = 8) mg/kg. There was no evidence of cidofovir accumulation after 4 weeks of repeated administration of 3 mg/kg/week (n = 5) without probenecid. In patients with normal renal function, approximately 80 to 100% of the VISTIDE dose was recovered unchanged in urine within 24 hr (n = 27). The renal clearance of cidofovir was greater than creatinine clearance, indicating renal tubular secretion contributes to the elimination of cidofovir.
The pharmacokinetics of cidofovir administered with probenecid were evaluated in 12 HIV-infected patients with or without asymptomatic CMV infection and 10 patients with relapsing CMV retinitis. Dose-independent pharmacokinetics were observed for cidofovir, administered with probenecid, after one hr infusions of 3.0 (n = 12), 5.0 (n = 6), and 7.5 (n = 4) mg/kg (See TABLE 2). Approximately 70 to 85% of the VISTIDE dose administered with concomitant probenecid was excreted as unchanged drug within 24 hr. When VISTIDE was administered with probenecid, the renal clearance of cidofovir was reduced to a level consistent with creatinine clearance, suggesting that probenecid blocks active renal tubular secretion of cidofovir.
Nonclinical Toxicology
There is limited information regarding Cidofovir Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Cidofovir Clinical Studies in the drug label.
How Supplied
There is limited information regarding Cidofovir How Supplied in the drug label.
Storage
There is limited information regarding Cidofovir Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Cidofovir |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Cidofovir |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Cidofovir Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Cidofovir interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Cidofovir Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Cidofovir Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Cundy, Kenneth C. "Clinical Pharmacokinetics of the Antiviral Nucleotide Analogues Cidofovir and Adefovir." Clinical Pharmacokinetics 36.2 (1999): 127-43.
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
Overview
Cidofovir is an injectable antiviral medication for the treatment of cytomegalovirus (CMV) retinitis in patients with AIDS. It suppresses CMV replication by selective inhibition of viral DNA synthesis.
Cidofovir demonstrated a statistically significant effect in delaying the progression of CMV retinitis lesions in newly diagnosed patients, as well as in previously treated patients who had failed other therapies. Maintenance therapy with cidofovir involves an infusion only once every two weeks, making it a convenient treatment option. Because dosing is relatively infrequent, a permanent catheter is not necessary for infusions. The major side effect of cidofovir is that it can be nephrotoxic.
Category
Antiviral
US Brand Names
CIDOFOVIR®
FDA Package Insert
Description | Clinical Pharmacology | Microbiology | Indications and Usage | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Overdosage | Clinical Studies | Dosage and Administration | How Supplied | Labels and Packages
Mechanism of Action
Cidofovir suppresses cytomegalovirus (CMV) replication by selective inhibition of viral DNA synthesis. Biochemical data support selective inhibition of CMV DNA polymerase by cidofovir diphosphate, the active intracellular metabolite of cidofovir. Cidofovir diphosphate inhibits herpesvirus polymerases at concentrations that are 8- to 600-fold lower than those needed to inhibit human cellular DNA polymerases alpha, beta, and gamma1, 2, 3. Incorporation of cidofovir into the growing viral DNA chain results in reductions in the rate of viral DNA synthesis.