COVID-19-associated pulmonary hypertension: Difference between revisions
No edit summary |
|||
| (17 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
{{Main article|COVID-19}} | {{Main article|COVID-19}} | ||
{{SI}} | {{SI}} | ||
'''For COVID-19 frequently asked inpatient questions, click [[COVID-19 frequently asked inpatient questions|here]]'''<br> | |||
'''For COVID-19 frequently asked outpatient questions, click [[COVID-19 frequently asked outpatient questions|here]]'''<br>'''For COVID-19 patient information, click [[COVID-19 (patient information)|here]]''' | |||
{{CMG}}; {{AE}} [[User:Sara Zand|Sara Zand, M.D.]] | {{CMG}}; {{AE}} [[User:Sara Zand|Sara Zand, M.D.]] | ||
{{SK}} | {{SK}} Pulmonary hypertension, PH, COVID-19, SARS-COV-2, ARDS | ||
==Overview== | ==Overview== | ||
Pulmonary hypertension (PH) is determined as an increase in [[ | [[Pulmonary hypertension]] ([[PH]]) is determined as an increase in mean [[pulmonary arterial pressure]] (mPAP) of 25 mm Hg or greater at rest. It occurs due to [[pulmonary arterial remodeling]] and [[vasoconstriction]] prompting an increase in [[pulmonary artery pressure]] and finally leading to [[right heart failure]]. Few cases of [[Covid-19]] with PH were found and it seems due to [[keeping social distance]] and quarantine, the number of cases is underestimated. [[Pulmonary hypertension]] is a rare disease and studies about [[PH]] during [[SARS-CoV]] disease in 2003 implied the role of [[inflammation]] in this process. | ||
==Historical Perspective== | ==Historical Perspective== | ||
| Line 46: | Line 50: | ||
#[[Hypoxia]] following diffuse alveolar and interestitial inflammation. [[Hypoxia]] may induce endothelial dysfunction and activation of [[coagulation cascade]] in small vessles.<ref name="TenPinsky2002">{{cite journal|last1=Ten|first1=Vadim S.|last2=Pinsky|first2=David J.|title=Endothelial response to hypoxia: physiologic adaptation and pathologic dysfunction|journal=Current Opinion in Critical Care|volume=8|issue=3|year=2002|pages=242–250|issn=1070-5295|doi=10.1097/00075198-200206000-00008}}</ref> | #[[Hypoxia]] following diffuse alveolar and interestitial inflammation. [[Hypoxia]] may induce endothelial dysfunction and activation of [[coagulation cascade]] in small vessles.<ref name="TenPinsky2002">{{cite journal|last1=Ten|first1=Vadim S.|last2=Pinsky|first2=David J.|title=Endothelial response to hypoxia: physiologic adaptation and pathologic dysfunction|journal=Current Opinion in Critical Care|volume=8|issue=3|year=2002|pages=242–250|issn=1070-5295|doi=10.1097/00075198-200206000-00008}}</ref> | ||
#[[ACE2]] receptor expression downregulation after attaching the [[sparkle site|spike site]] of [[COVID-19]] to [[pneumocytes type2]]. | #[[ACE2]] receptor expression downregulation after attaching the [[sparkle site|spike site]] of [[COVID-19]] to [[pneumocytes type2]]. | ||
# Activation of the innate coagulation cascade | # Activation of the innate coagulation cascade in older age. | ||
#[[Mechanical ventilation]] may induce immune micro thrombosis in small arteries.<ref name="pmid23222502">{{cite journal |vauthors=Engelmann B, Massberg S |title=Thrombosis as an intravascular effector of innate immunity |journal=Nat. Rev. Immunol. |volume=13 |issue=1 |pages=34–45 |date=January 2013 |pmid=23222502 |doi=10.1038/nri3345 |url=}}</ref> | #[[Mechanical ventilation]] may induce immune micro thrombosis in small arteries.<ref name="pmid23222502">{{cite journal |vauthors=Engelmann B, Massberg S |title=Thrombosis as an intravascular effector of innate immunity |journal=Nat. Rev. Immunol. |volume=13 |issue=1 |pages=34–45 |date=January 2013 |pmid=23222502 |doi=10.1038/nri3345 |url=}}</ref> | ||
#[[Bacterial superinfection.]] | #[[Bacterial superinfection.]] | ||
| Line 52: | Line 56: | ||
==Differentiating COVID-19-associated pulmonary hypertension from other Diseases== | ==Differentiating COVID-19-associated pulmonary hypertension from other Diseases== | ||
* Pulmonary intravascular coagulopathy causing pulmonary hypertention in [[COVID-19]] must be differentiated from [[disseminated intravascular coagulation(DIC)|disseminated intravascular coagulation (DIC)]] based on clinical features and lab data:<ref name=" | * Pulmonary intravascular coagulopathy causing pulmonary hypertention in [[COVID-19]] must be differentiated from [[disseminated intravascular coagulation(DIC)|disseminated intravascular coagulation (DIC)]] based on clinical features and lab data:<ref name="McGonagleSharif2020">{{cite journal|last1=McGonagle|first1=Dennis|last2=Sharif|first2=Kassem|last3=O'Regan|first3=Anthony|last4=Bridgewood|first4=Charlie|title=The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease|journal=Autoimmunity Reviews|volume=19|issue=6|year=2020|pages=102537|issn=15689972|doi=10.1016/j.autrev.2020.102537}}</ref> | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 67: | Line 72: | ||
|100% | |100% | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Thrombosis | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Thrombosis]] | ||
|Multi-organ clotting | |Multi-organ clotting | ||
|Mainly lung | |Mainly lung | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Bleeding | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Bleeding]] | ||
|Generalised | |Generalised | ||
|Intrapulmonary microhaemorrhage | |Intrapulmonary microhaemorrhage | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Liver function | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Liver]] function | ||
|Decreased | |Decreased fibrinogen and other clotting factors; increased transaminase +++ | ||
|Preservation of liver synthetic function; +/− | |Preservation of liver synthetic function; +/− | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Anemia | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Anemia]] | ||
| +++ | | +++ | ||
|− | |− | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Thrombocytopenia | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Thrombocytopenia]] | ||
| +++ | | +++ | ||
|Normal or low | |Normal or low | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" | | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[cytopenia]] | ||
| ++ | | ++ | ||
|No | |No.may be lymphopenia is a finding of COVID-19 | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Creatine kinase | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Creatine kinase-myocardial type|Creatine kinase]] | ||
| + | | + | ||
| + | | + | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Troponin T | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Troponin T]] | ||
| + | | + | ||
| ++ | | ++ | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Elevated prothrombin time or activated partial thromboplastin time | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Elevated [[prothrombin time]] or activated [[partial thromboplastin time]] | ||
| +++/+++ | | +++/+++ | ||
| + or normal | | + or normal | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Fibrinogen levels | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Fibrinogen]] levels | ||
|Decreased | |Decreased | ||
|Normal or slight increase | |Normal or slight increase | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Fibrin degradation products or D-dimer | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Fibrin degradation products]] or [[D-dimer]] | ||
|Increased | |Increased | ||
|Increased | |Increased | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |C-reactive protein | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[C-reactive protein]] | ||
|Elevated | |Elevated | ||
|Elevated | |Elevated | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Ferritin elevation | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Ferritin]] elevation | ||
| +++ | | +++ | ||
|Elevated | |Elevated | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Hypercytokinaemia | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Hypercytokinaemia]] | ||
| +++ | | +++ | ||
| ++ | | ++ | ||
| Line 126: | Line 131: | ||
==Epidemiology and Demographics== | ==Epidemiology and Demographics== | ||
* Data on incidence | * Data on the incidence of pulmonary hypertension in [[COVID-19]] patients is limited. | ||
*There is no racial predilection to pulmonary hypertension in [[COVID-19]]. | *There is no racial predilection to pulmonary hypertension in [[COVID-19]]. | ||
* Male are more commonly affected by [[COVID-19]] than female, therefore, the prevalence of [[pulmonary hypertension]] induced by [[Covid-19]] is higher in the male gender. | * Male are more commonly affected by [[COVID-19]] than female, therefore, the prevalence of [[pulmonary hypertension]] induced by [[Covid-19]] is higher in the male gender. | ||
| Line 230: | Line 235: | ||
===MRI=== | ===MRI=== | ||
*[[Cardiac MRI]] is one of the most accurate method in the diagnosis of pulmonary hypertension. Findings on MRI | *[[Cardiac MRI]] is one of the most accurate method in the diagnosis of pulmonary hypertension. Findings on MRI for evaluation of pulmonary hypertension include :<ref name="pmid23168063">{{cite journal |vauthors=Frazier AA, Burke AP |title=The imaging of pulmonary hypertension |journal=Semin. Ultrasound CT MR |volume=33 |issue=6 |pages=535–51 |date=December 2012 |pmid=23168063 |doi=10.1053/j.sult.2012.06.002 |url=}}</ref> | ||
**Assessment of the anatomy of the [[pulmonary arteries]]. | **Assessment of the anatomy of the [[pulmonary arteries]]. | ||
**Assessment of [[pulmonary blood flow]]. | **Assessment of [[pulmonary blood flow]]. | ||
| Line 236: | Line 241: | ||
===Other Imaging Findings=== | ===Other Imaging Findings=== | ||
*[[Perfusion ventilation scan]] may be helpful in the diagnosis of chronic thromboembolic pulmonary hypertension without ventilation portion due to difficulty in disinfecting the ventilation system in [[COVID-19]] pandemic. | *[[Perfusion ventilation scan]] may be helpful in the diagnosis of chronic thromboembolic pulmonary hypertension without the ventilation portion due to difficulty in disinfecting the ventilation system in [[COVID-19]] pandemic. | ||
*If [[lung perfusion image]] is normal, [[chronic thromboembolism]] is ruled out and further invasive [[catheterization]] | *If [[lung perfusion image]] is normal, [[chronic thromboembolism]] is ruled out and further invasive [[catheterization]] may be avoided.<ref name="GalièHumbert2016">{{cite journal|last1=Galiè|first1=Nazzareno|last2=Humbert|first2=Marc|last3=Vachiery|first3=Jean-Luc|last4=Gibbs|first4=Simon|last5=Lang|first5=Irene|last6=Torbicki|first6=Adam|last7=Simonneau|first7=Gérald|last8=Peacock|first8=Andrew|last9=Vonk Noordegraaf|first9=Anton|last10=Beghetti|first10=Maurice|last11=Ghofrani|first11=Ardeschir|last12=Gomez Sanchez|first12=Miguel Angel|last13=Hansmann|first13=Georg|last14=Klepetko|first14=Walter|last15=Lancellotti|first15=Patrizio|last16=Matucci|first16=Marco|last17=McDonagh|first17=Theresa|last18=Pierard|first18=Luc A.|last19=Trindade|first19=Pedro T.|last20=Zompatori|first20=Maurizio|last21=Hoeper|first21=Marius|title=2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension|journal=European Heart Journal|volume=37|issue=1|year=2016|pages=67–119|issn=0195-668X|doi=10.1093/eurheartj/ehv317}}</ref> | ||
===Other Diagnostic Studies=== | ===Other Diagnostic Studies=== | ||
| Line 248: | Line 253: | ||
#[[Pulmonary vasodilation]].[[Nitric oxide]] has antiviral and anti inflammatory effect in [[SARS-CoV]] .<ref name="pmid15546092">{{cite journal |vauthors=Chen L, Liu P, Gao H, Sun B, Chao D, Wang F, Zhu Y, Hedenstierna G, Wang CG |title=Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: a rescue trial in Beijing |journal=Clin. Infect. Dis. |volume=39 |issue=10 |pages=1531–5 |date=November 2004 |pmid=15546092 |pmc=7107896 |doi=10.1086/425357 |url=}}</ref> | #[[Pulmonary vasodilation]].[[Nitric oxide]] has antiviral and anti inflammatory effect in [[SARS-CoV]] .<ref name="pmid15546092">{{cite journal |vauthors=Chen L, Liu P, Gao H, Sun B, Chao D, Wang F, Zhu Y, Hedenstierna G, Wang CG |title=Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: a rescue trial in Beijing |journal=Clin. Infect. Dis. |volume=39 |issue=10 |pages=1531–5 |date=November 2004 |pmid=15546092 |pmc=7107896 |doi=10.1086/425357 |url=}}</ref> | ||
#Supplement [[oxygen]] for correction of [[hypoxia]] and prevention of pulmonary vasoconstriction to maintain [[oxygen saturation]] above 92%. | #Supplement [[oxygen]] for correction of [[hypoxia]] and prevention of pulmonary vasoconstriction to maintain [[oxygen saturation]] above 92%. | ||
#Avoidance of inhaled | #Avoidance of inhaled prostacyclin for prevention of spreading [[COVID-19]] and using the parenteral form for the protection of health care providers. | ||
#[[Endothelin receptor antagonist]] agents. | #[[Endothelin receptor antagonist]] agents. | ||
#[[Anticoagulation therapy]] if there is evidence of thromboembolic mechanism.<ref name="pmid32299776">{{cite journal |vauthors=Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J |title=Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases |journal=Transl Res |volume=220 |issue= |pages=1–13 |date=June 2020 |pmid=32299776 |pmc=7158248 |doi=10.1016/j.trsl.2020.04.007 |url=}}</ref> | #[[Anticoagulation therapy]] if there is evidence of thromboembolic mechanism.<ref name="pmid32299776">{{cite journal |vauthors=Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J |title=Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases |journal=Transl Res |volume=220 |issue= |pages=1–13 |date=June 2020 |pmid=32299776 |pmc=7158248 |doi=10.1016/j.trsl.2020.04.007 |url=}}</ref> | ||
# Correction of [[hypotension]] with fluild and [[inotropic agent]]<nowiki/>s in order to avoid [[RV coronary perfusion|decreased RV coronary perfusion]] and [[RV ejection]]. | # Correction of [[hypotension]] with fluild and [[inotropic agent]]<nowiki/>s in order to avoid [[RV coronary perfusion|decreased RV coronary perfusion]] and [[RV ejection fraction]]. | ||
# Correction of acidosis, [[hypercarbia]], [[hypothermia]], [[hypervolemia]]. | # Correction of acidosis, [[hypercarbia]], [[hypothermia]], [[hypervolemia]]. | ||
#[[Intubation]] is not recommended due to the effect of [[positive pressure ventilation]] in increasing [[RV preload]] and also [[vasodilatory effect]] of [[sedation agents]] impending [[systemic hypotension]] and hemodynamic [[collapse]]. | #[[Intubation]] is not recommended due to the effect of [[positive pressure ventilation]] in increasing [[RV preload]] and also [[vasodilatory effect]] of [[sedation agents]] impending [[systemic hypotension]] and hemodynamic [[collapse]]. | ||
| Line 267: | Line 272: | ||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
[[Category:Up-To-Date]] | |||
{{WikiDoc Help Menu}} | |||
{{WikiDoc Sources}} | |||
Latest revision as of 08:31, 11 November 2021
For COVID-19 frequently asked inpatient questions, click here
For COVID-19 frequently asked outpatient questions, click here
For COVID-19 patient information, click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]; Associate Editor(s)-in-Chief: Sara Zand, M.D.
Synonyms and keywords: Pulmonary hypertension, PH, COVID-19, SARS-COV-2, ARDS
Overview
Pulmonary hypertension (PH) is determined as an increase in mean pulmonary arterial pressure (mPAP) of 25 mm Hg or greater at rest. It occurs due to pulmonary arterial remodeling and vasoconstriction prompting an increase in pulmonary artery pressure and finally leading to right heart failure. Few cases of Covid-19 with PH were found and it seems due to keeping social distance and quarantine, the number of cases is underestimated. Pulmonary hypertension is a rare disease and studies about PH during SARS-CoV disease in 2003 implied the role of inflammation in this process.
Historical Perspective
- In 2003, the association between corona infection and pulmonary hypertension was established during SARS-CoV epidemic.
Classification
- Pulmonary hypertension in COVID-19 may be classified into two subtypes:
- Pulmonary hypertension due to lung disease or hypoxia.
- Microvascular thromboembolic pulmonary hypertension.
Pathophysiology
- The SARS-CoV-2 and SARS-CoV virus genomes are highly similar, and patients infected with these viruses have common pathological features.[1]
- The pathogenesis of PH in COVID-19 is characterized by pulmonary vasoconstriction due to lack of ACE2 and pulmonary microthromboembolism due to local endothelial cell dysfunction.
- Renin angiotensin system (RAS) is responsible for hemostasis of blood pressure and electrolyte balance and inflammatory response.
- Renin is a protease that is generated in the kidney and cleaves angiotensinogen to angiotensin1, Then angiotensin converting enzyme (ACE) cleaves angiotensin 1 to angiotensin 2.
- Angiotensin 2 is a key factor of RAS and has two receptors including type 1 and type 2.
- Unbalance between ACE/Ang II/AT1R pathway and ACE2/Ang (1-7) receptor pathway in the RAS system will induce multi-system inflammation.[2]
- Angiotensin-converting enzyme 2 (ACE2), and neprilysin hydrolyze angiotensin 2 to anti inflammatory agents including Ang1–7, Ang III, Ang IV, and Ang A.[3]
- Angiotensin-converting enzyme 2 (ACE2) was a receptor of spike protein on SARS corona virus in epithelial cell and after attaching virus the activity of enzyme(ACE2) was decreased and then virus spread quickly.[4][5]
- Lack of ACE2 causes elevation in angiotensin 2 level causing vascular permeability and lung edema and neutrophil infiltration and further lung deterioration.
- ACE2 has anti inflammation effect and protected the lung from acute lung injury.[6]
- Phosphorilized ACE2 is a much more stable form in which it converts angiotensin 2 to angiotensin 1-7 and increases the bioavailability of endothelial nitric oxide synthase-derived NO.
- Lack of phosphorilized ACE2 causes vasoconstriction and pulmonary hypertension.[7]
- Nitric oxide inhalation for SARS-corona patients was correlated with vasodilation and relaxation of pulmonary artery, reduction in pulmonary artery pressure and improvement in arterial oxygenation.[8]
- Endothelin-1 caused downregulated ACE2 expression in lung epithelial cells and reduced pulmonary vasoconstriction.[9]
- On microscopic histopathological analysis, pulmonary wall edema,hyalin thrombosis, inflammatory cell infiltration of pulmonary microvasculature , vessle thrombosis due to diffuse alveolar damage and septal inflammation are characteristic findings of PH in COVID-19.[10]
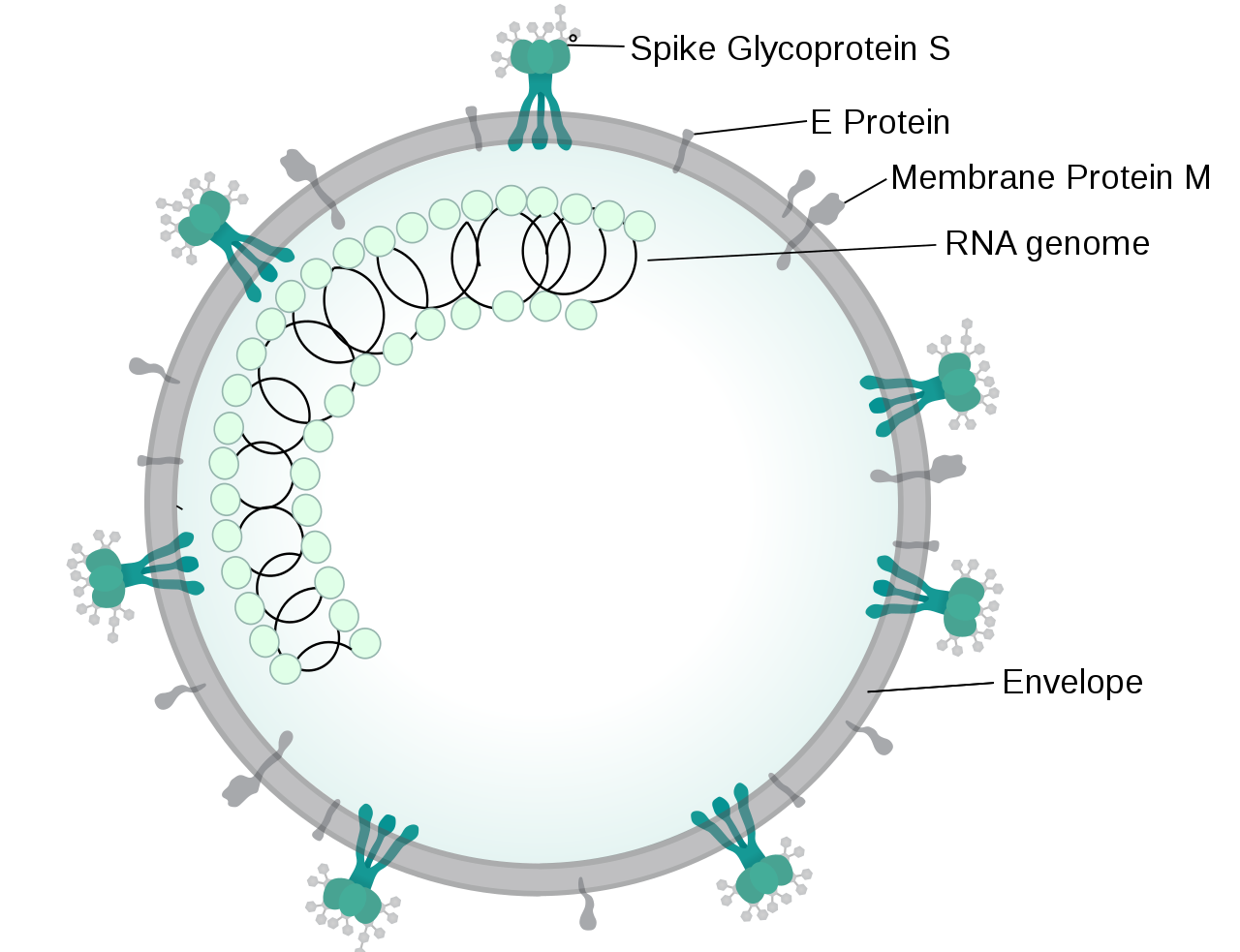 |
Causes
- Factors contributing to the microthrombi formation in the pulmonary artery in COVID-19 include:
- Hypoxia following diffuse alveolar and interestitial inflammation. Hypoxia may induce endothelial dysfunction and activation of coagulation cascade in small vessles.[11]
- ACE2 receptor expression downregulation after attaching the spike site of COVID-19 to pneumocytes type2.
- Activation of the innate coagulation cascade in older age.
- Mechanical ventilation may induce immune micro thrombosis in small arteries.[12]
- Bacterial superinfection.
Differentiating COVID-19-associated pulmonary hypertension from other Diseases
- Pulmonary intravascular coagulopathy causing pulmonary hypertention in COVID-19 must be differentiated from disseminated intravascular coagulation (DIC) based on clinical features and lab data:[13]
| Disseminated intravascular coagulopathy | Pulmonary intravascular coagulopathy | |
|---|---|---|
| Onset | Acute | Subacute |
| Pulmonary involvement (%) | 50% | 100% |
| Thrombosis | Multi-organ clotting | Mainly lung |
| Bleeding | Generalised | Intrapulmonary microhaemorrhage |
| Liver function | Decreased fibrinogen and other clotting factors; increased transaminase +++ | Preservation of liver synthetic function; +/− |
| Anemia | +++ | − |
| Thrombocytopenia | +++ | Normal or low |
| cytopenia | ++ | No.may be lymphopenia is a finding of COVID-19 |
| Creatine kinase | + | + |
| Troponin T | + | ++ |
| Elevated prothrombin time or activated partial thromboplastin time | +++/+++ | + or normal |
| Fibrinogen levels | Decreased | Normal or slight increase |
| Fibrin degradation products or D-dimer | Increased | Increased |
| C-reactive protein | Elevated | Elevated |
| Ferritin elevation | +++ | Elevated |
| Hypercytokinaemia | +++ | ++ |
Epidemiology and Demographics
- Data on the incidence of pulmonary hypertension in COVID-19 patients is limited.
- There is no racial predilection to pulmonary hypertension in COVID-19.
- Male are more commonly affected by COVID-19 than female, therefore, the prevalence of pulmonary hypertension induced by Covid-19 is higher in the male gender.
Risk Factors
- Common risk factors in the development of pulmonary hypertention in COVID-19 include the following:[14]
Screening
- There is insufficient evidence to recommend routine screening for pulmonary hypertension in COVID-19.
Natural History, Complications, and Prognosis
- Severe COVID-19 infection induces cytokine storm which leads to activation of the coagulation cascade and thrombotic process.
- Inflammatory markers including IL-1,IL-6 and tumor necrosis factor and ferritin concentration, cause pulmonary endothelial dysfunction and thromboinflammatory process.[15]
- Hypoxia in COVID-19 pneumonia will cause endothelial dysfunction and expression of active tissue factor on endothelium, macrophage, neutrophils, and finally activation of coagulation cascade and reduction of fibrinolysis and plasminogen activation inhibitor 1.
- Thrombosis and hemorrhage occur in small vessels of the lung and thrombin generation and fibrin deposition enhances in the bronchoalveolar system.
- These show the severity of inflammation.
- D-dimer level correlated with severe COVID-19 and indicates activation fibrinolysis and plasmin generation.[16]
- COVID-19 downregulates ACE2 on pneumocytes type2 which are adjusted pulmonary vascular bed, then vasculopathy and thrombosis happens.
- Prognosis is generally poor in older patients and in patients with high level of fibrin degeredated factors, including, D-dimer and cardiac troponin T due to right ventricular failure.[17]
- COVID-19 associated pulmonary hypertension is a chronic progressive disease with a high mortality rate.
Diagnosis
Diagnostic Study of Choice
- The diagnosis of pulmonary hypertension is made when at least three of the following diagnostic criteria in right heart catheterization (RHC) are met:
- Mean PAP≥25 mmHg,
- Pulmonary artery wedge pressure≤15 mmHg.
- Pulmonary vascular resistance>3 wood units.
History and Symptoms
- The most common symptoms of pulmonary hypertension include:
- Less common symptoms include:
Physical Examination
- Physical examination in PH may be remarkable for:
- Rales
- Dullness or decreased breath sound due to pulmonary congestion or effusion.
- Central cyanosis due to hypoxia.
- Holosystolic murmur with increased intensity during inspiration due to tricuspid regurgitation (TR).
- Diastolic murmur due to pulmonary regurgitation.
- Hepatojugular reflux.
- Right ventricular S3 due to RV dysfunction.
- Distention of jugular veins due to RV dysfunction and TR
- Peripheral edema and ascites.
- Low blood Pressure.
- Diminished pulse pressure.
- Cool extremities due to reduced cardiac output and peripheral vasoconstriction.
- Abdominal distention
- Lower extremity edema (seen in advanced disease and right ventricular failure).
- Racing pulse
Laboratory Findings
- Laboratory findings consistent with the diagnosis of pulmonary hypertension in COVID-19 include:
- Increased D-dimer (due to pulmonary vascular bed thrombosis with fibrinolysis).
- Elevated concentration of cardiac enzymes due to right ventricular strain induced by pulmonary hypertension.
- Normal fibrinogen and platelet level.
Electrocardiogram
- An ECG may be helpful in the diagnosis of pulmonary hypertension. Findings on an ECG suggestive pulmonary hypertension include:
X-ray
- Chest x-ray may be helpful in the diagnosis of pulmonary hypertension in COVID-19. Findings on chest x-ray suggestive of pulmonary hypertension include:
- Enlarged main pulmonary artery.
- Prunning or attenuation of the peripheral vasculature.
- Right ventricular enlargement especially in lateral view.
- With other evidence of lung involvement in COVID-19.
Echocardiography or Ultrasound
- Echocardiography is the first modality in the diagnosis of pulmonary hypertension. Findings on an echocardiography suggestive of pulmonary hypertension include:
- Right atrial enlargement
- Right ventricular enlargement and dysfunction
- Small left side chambers
- Interventricular setal flattening
- Tricuspid regurgitation
CT scan
- Chest CT scan even unenhanced may be helpful in the diagnosis of pulmonary hypertension in COVID-19.. Findings on CT scan suggestive pulmonary hypertension in COVID-19 in comparison with baseline chest CT scan include:[18]
- Pulmonary artery dilation above 27mm in women and 29mm in men.
- Increased median Pulmonary Artery/Aorta ratio from 26mm to 31mm after SARS-COVID infection.
- In one study, pulmonary artery dilation was correlated with high level of D-dimer and pulmonary artery thrombosis and poor outcome in COVID-19.[19]
MRI
- Cardiac MRI is one of the most accurate method in the diagnosis of pulmonary hypertension. Findings on MRI for evaluation of pulmonary hypertension include :[20]
- Assessment of the anatomy of the pulmonary arteries.
- Assessment of pulmonary blood flow.
- Assessment of right ventricular size, morphology, and function.
Other Imaging Findings
- Perfusion ventilation scan may be helpful in the diagnosis of chronic thromboembolic pulmonary hypertension without the ventilation portion due to difficulty in disinfecting the ventilation system in COVID-19 pandemic.
- If lung perfusion image is normal, chronic thromboembolism is ruled out and further invasive catheterization may be avoided.[21]
Other Diagnostic Studies
- There are no other diagnostic studies associated with pulmonary hypertension in COVID-19.
Treatment
Medical Therapy
- The mainstay of therapy for pulmonary hypertension in Covid-19 including:[22][23]
- Pulmonary vasodilation.Nitric oxide has antiviral and anti inflammatory effect in SARS-CoV .[24]
- Supplement oxygen for correction of hypoxia and prevention of pulmonary vasoconstriction to maintain oxygen saturation above 92%.
- Avoidance of inhaled prostacyclin for prevention of spreading COVID-19 and using the parenteral form for the protection of health care providers.
- Endothelin receptor antagonist agents.
- Anticoagulation therapy if there is evidence of thromboembolic mechanism.[25]
- Correction of hypotension with fluild and inotropic agents in order to avoid decreased RV coronary perfusion and RV ejection fraction.
- Correction of acidosis, hypercarbia, hypothermia, hypervolemia.
- Intubation is not recommended due to the effect of positive pressure ventilation in increasing RV preload and also vasodilatory effect of sedation agents impending systemic hypotension and hemodynamic collapse.
- If intubation is indicated, a vasoactive agent should be given before anesthesia. Etomidate is recommended for general anesthesia due to little effect on cardiac contractility and vascular tone.
- Ventilator should be set with low tidal volumes and moderate positive end-expiratory pressure for minimum airway pressure and sufficient oxygenation and ventilation.
Surgery
- Surgical intervention is not recommended for the management of pulmonary hypertension in COVID-19.
Primary Prevention
- Effective measures for the primary prevention of PH and COVID-19 include keeping social distancing and maintaining the medication used for pulmonary hypertension.
Secondary Prevention
- There are no established measures for the secondary prevention of pulmonary hypertension in COVID-19.
References
- ↑ Zhu, Na; Zhang, Dingyu; Wang, Wenling; Li, Xingwang; Yang, Bo; Song, Jingdong; Zhao, Xiang; Huang, Baoying; Shi, Weifeng; Lu, Roujian; Niu, Peihua; Zhan, Faxian; Ma, Xuejun; Wang, Dayan; Xu, Wenbo; Wu, Guizhen; Gao, George F.; Tan, Wenjie (2020). "A Novel Coronavirus from Patients with Pneumonia in China, 2019". New England Journal of Medicine. 382 (8): 727–733. doi:10.1056/NEJMoa2001017. ISSN 0028-4793.
- ↑ Konishi H, Kuroda S, Inada Y, Fujisawa Y (March 1994). "Novel subtype of human angiotensin II type 1 receptor: cDNA cloning and expression". Biochem. Biophys. Res. Commun. 199 (2): 467–74. doi:10.1006/bbrc.1994.1252. PMID 8135787.
- ↑ Chappell MC (October 2012). "Nonclassical renin-angiotensin system and renal function". Compr Physiol. 2 (4): 2733–52. doi:10.1002/cphy.c120002. PMC 4186703. PMID 23720263.
- ↑ Li, Wenhui; Moore, Michael J.; Vasilieva, Natalya; Sui, Jianhua; Wong, Swee Kee; Berne, Michael A.; Somasundaran, Mohan; Sullivan, John L.; Luzuriaga, Katherine; Greenough, Thomas C.; Choe, Hyeryun; Farzan, Michael (2003). "Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus". Nature. 426 (6965): 450–454. doi:10.1038/nature02145. ISSN 0028-0836.
- ↑ Glowacka I, Bertram S, Herzog P, Pfefferle S, Steffen I, Muench MO, Simmons G, Hofmann H, Kuri T, Weber F, Eichler J, Drosten C, Pöhlmann S (January 2010). "Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63". J. Virol. 84 (2): 1198–205. doi:10.1128/JVI.01248-09. PMC 2798380. PMID 19864379.
- ↑ Imai, Yumiko; Kuba, Keiji; Rao, Shuan; Huan, Yi; Guo, Feng; Guan, Bin; Yang, Peng; Sarao, Renu; Wada, Teiji; Leong-Poi, Howard; Crackower, Michael A.; Fukamizu, Akiyoshi; Hui, Chi-Chung; Hein, Lutz; Uhlig, Stefan; Slutsky, Arthur S.; Jiang, Chengyu; Penninger, Josef M. (2005). "Angiotensin-converting enzyme 2 protects from severe acute lung failure". Nature. 436 (7047): 112–116. doi:10.1038/nature03712. ISSN 0028-0836.
- ↑ Zhang, Jiao; Dong, Jianjie; Martin, Marcy; He, Ming; Gongol, Brendan; Marin, Traci L.; Chen, Lili; Shi, Xinxing; Yin, Yanjun; Shang, Fenqing; Wu, Yan; Huang, Hsi-Yuan; Zhang, Jin; Zhang, Yu; Kang, Jian; Moya, Esteban A.; Huang, Hsien-Da; Powell, Frank L.; Chen, Zhen; Thistlethwaite, Patricia A.; Yuan, Zu-Yi; Shyy, John Y.-J. (2018). "AMP-activated Protein Kinase Phosphorylation of Angiotensin-Converting Enzyme 2 in Endothelium Mitigates Pulmonary Hypertension". American Journal of Respiratory and Critical Care Medicine. 198 (4): 509–520. doi:10.1164/rccm.201712-2570OC. ISSN 1073-449X.
- ↑ Chen, L.; Liu, P.; Gao, H.; Sun, B.; Chao, D.; Wang, F.; Zhu, Y.; Hedenstierna, G.; Wang, C. G. (2004). "Inhalation of Nitric Oxide in the Treatment of Severe Acute Respiratory Syndrome: A Rescue Trial in Beijing". Clinical Infectious Diseases. 39 (10): 1531–1535. doi:10.1086/425357. ISSN 1058-4838.
- ↑ Zhang H, Li Y, Zeng Y, Wu R, Ou J (2013). "Endothelin-1 downregulates angiotensin-converting enzyme-2 expression in human bronchial epithelial cells". Pharmacology. 91 (5–6): 297–304. doi:10.1159/000350395. PMID 23751363.
- ↑ Fox, Sharon E.; Akmatbekov, Aibek; Harbert, Jack L.; Li, Guang; Brown, J. Quincy; Vander Heide, Richard S. (2020). doi:10.1101/2020.04.06.20050575. Missing or empty
|title=(help) - ↑ Ten, Vadim S.; Pinsky, David J. (2002). "Endothelial response to hypoxia: physiologic adaptation and pathologic dysfunction". Current Opinion in Critical Care. 8 (3): 242–250. doi:10.1097/00075198-200206000-00008. ISSN 1070-5295.
- ↑ Engelmann B, Massberg S (January 2013). "Thrombosis as an intravascular effector of innate immunity". Nat. Rev. Immunol. 13 (1): 34–45. doi:10.1038/nri3345. PMID 23222502.
- ↑ McGonagle, Dennis; Sharif, Kassem; O'Regan, Anthony; Bridgewood, Charlie (2020). "The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease". Autoimmunity Reviews. 19 (6): 102537. doi:10.1016/j.autrev.2020.102537. ISSN 1568-9972.
- ↑ McGonagle, Dennis; O'Donnell, James S; Sharif, Kassem; Emery, Paul; Bridgewood, Charles (2020). "Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia". The Lancet Rheumatology. 2 (7): e437–e445. doi:10.1016/S2665-9913(20)30121-1. ISSN 2665-9913.
- ↑ Levi M, van der Poll T (January 2017). "Coagulation and sepsis". Thromb. Res. 149: 38–44. doi:10.1016/j.thromres.2016.11.007. PMID 27886531.
- ↑ Ji HL, Zhao R, Matalon S, Matthay MA (July 2020). "Elevated Plasmin(ogen) as a Common Risk Factor for COVID-19 Susceptibility". Physiol. Rev. 100 (3): 1065–1075. doi:10.1152/physrev.00013.2020. PMC 7191627 Check
|pmc=value (help). PMID 32216698 Check|pmid=value (help). - ↑ Zhou, Fei; Yu, Ting; Du, Ronghui; Fan, Guohui; Liu, Ying; Liu, Zhibo; Xiang, Jie; Wang, Yeming; Song, Bin; Gu, Xiaoying; Guan, Lulu; Wei, Yuan; Li, Hui; Wu, Xudong; Xu, Jiuyang; Tu, Shengjin; Zhang, Yi; Chen, Hua; Cao, Bin (2020). "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study". The Lancet. 395 (10229): 1054–1062. doi:10.1016/S0140-6736(20)30566-3. ISSN 0140-6736.
- ↑ Spagnolo, Pietro; Cozzi, Andrea; Foà, Riccardo Alessandro; Spinazzola, Angelo; Monfardini, Lorenzo; Bnà, Claudio; Alì, Marco; Schiaffino, Simone; Sardanelli, Francesco (2020). "CT-derived pulmonary vascular metrics and clinical outcome in COVID-19 patients". Quantitative Imaging in Medicine and Surgery. 10 (6): 1325–1333. doi:10.21037/qims-20-546. ISSN 2223-4292.
- ↑ Dolhnikoff, Marisa; Duarte‐Neto, Amaro Nunes; Almeida Monteiro, Renata Aparecida; Silva, Luiz Fernando Ferraz; Oliveira, Ellen Pierre; Saldiva, Paulo Hilário Nascimento; Mauad, Thais; Negri, Elnara Marcia (2020). "Pathological evidence of pulmonary thrombotic phenomena in severe COVID‐19". Journal of Thrombosis and Haemostasis. 18 (6): 1517–1519. doi:10.1111/jth.14844. ISSN 1538-7933.
- ↑ Frazier AA, Burke AP (December 2012). "The imaging of pulmonary hypertension". Semin. Ultrasound CT MR. 33 (6): 535–51. doi:10.1053/j.sult.2012.06.002. PMID 23168063.
- ↑ Galiè, Nazzareno; Humbert, Marc; Vachiery, Jean-Luc; Gibbs, Simon; Lang, Irene; Torbicki, Adam; Simonneau, Gérald; Peacock, Andrew; Vonk Noordegraaf, Anton; Beghetti, Maurice; Ghofrani, Ardeschir; Gomez Sanchez, Miguel Angel; Hansmann, Georg; Klepetko, Walter; Lancellotti, Patrizio; Matucci, Marco; McDonagh, Theresa; Pierard, Luc A.; Trindade, Pedro T.; Zompatori, Maurizio; Hoeper, Marius (2016). "2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension". European Heart Journal. 37 (1): 67–119. doi:10.1093/eurheartj/ehv317. ISSN 0195-668X.
- ↑ Gordon, Claire; Collard, Charles D; Pan, Wei (2010). "Intraoperative management of pulmonary hypertension and associated right heart failure". Current Opinion in Anaesthesiology. 23 (1): 49–56. doi:10.1097/ACO.0b013e3283346c51. ISSN 0952-7907.
- ↑ Pritts, Chad D; Pearl, Ronald G (2010). "Anesthesia for patients with pulmonary hypertension". Current Opinion in Anaesthesiology. 23 (3): 411–416. doi:10.1097/ACO.0b013e32833953fb. ISSN 0952-7907.
- ↑ Chen L, Liu P, Gao H, Sun B, Chao D, Wang F, Zhu Y, Hedenstierna G, Wang CG (November 2004). "Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: a rescue trial in Beijing". Clin. Infect. Dis. 39 (10): 1531–5. doi:10.1086/425357. PMC 7107896 Check
|pmc=value (help). PMID 15546092. - ↑ Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J (June 2020). "Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases". Transl Res. 220: 1–13. doi:10.1016/j.trsl.2020.04.007. PMC 7158248 Check
|pmc=value (help). PMID 32299776 Check|pmid=value (help).
.